Cases of Acute Pancreatitis
Case 1
32 years old male came to emergency department with complaint of:
- Upper abdominal pain X 3 days
- Vomiting X 2 episode (3 days back)
- Yellowish discoloration of urine and decreased urine output X 2 days
Vitals:
| Pulse rate | BP | RR | TPR | SpO2 | Triage Level |
| 102/min | 130/90 mmHg | 25/min | 97.2 F | 90% in RA | II |
K/C/O hypertension and not under medication; Normal bowel habit; No h/o LOC/abnormal body movement; No fever; No cough/difficulty breathing
G/C: agonized
Icterus: Present
P/A:
- Soft
- Tenderness over epigastrium and right hypochondrium
- Bowel sound present
Chest: B/L NVBS, B/L equal air entry, no added sounds
CVS: S1 S2 M0
CNS: Grossly Intact
Lab Reports:
1. Serum Amylase: 1524 IU/L (Normal 40-140 IU/L ; Reference – Medscape)
2. Serum Lipase: 396 IU/L (Normal 0-50 IU/L; Reference – Medscape)
3. Serum Creatinine: 5.1 mg/dl (Normal 0.5-1.2 mg/dl; Reference – Medscape)
4. BUN: 18.9 mg/dl (Normal 3-20 mg/dl; Reference – Medscape)
5. TLC: 19,900/cu.mm (Normal 4,000-12,000/cu.mm)
6. DLC: Neutrophil 94% (Normal 40-60%) ; Lymphocyte 6% (Normal 20-40%)
7. Hemoglobin: 17.1 g/dl (Normal 13-18 g/dl)
8. Platelets: 81,000/cu.mm (Normal 150,000-400,000/cu.mm)
Other Investigations:
1. INR: 1.07
2. Na+: 138 mEq/L
3. K+: 4.5 mEq/L
4. Total Bilirubin: 6.6 mg/dl
5. Direct Bilirubin: 2.2 mg/dl
6. SGOT (AST): 34 IU/L
7. SGPT (ALT): 20 IU/L
USG abdomen:
- Bulky pancreas with localized pancreatic collection likely pancreatitis
- Hepatomegaly and Splenomegaly
- Gall bladder sludge
- Bilateral increased cortical echo texture
- Ascites
- Left sided pleural effusion
CT Abdomen Revealed:
- Finding consistent with ascites with acute severe pancreatitis (modified CT Severity index 4+2+2=8 with moderate ascites)
- Mild hepatomegaly with fatty change in liver
- Minimal Left sided pleural effusion with Left lower lobe atelectasis
Diagnosis:
Severe Acute Pancreatitis (Alcoholic) with Acute Renal Failure
Disposition from ER:
Admitted to ICU after under following medications (after consultation from surgery and nephrology department):
- Inj. Imipenem 50mg iv stat then 250mg iv BD
- Inj. NS 3 pint IV over 24 hrs
- Inj. 5% dextrose 2 pint IV over 24 hrs
- Inj. Optineuron in 1 pint of NS OD
- Inj. Tramadol 50mg IV SOS
- Daily RFT
- Strict Renal Diet
At ICU:
- Inj. Tramadol replaced by Inj. Pethidine 50 mg IV SOS
- Regular vitals monitoring done
- Input/Output Charting done
- GRBS 4 hourly
- CVP Insertion done
Lab values some days later:
| 1.Serum Amylase: 97 IU/L 2.Serum Creatinine: 2.5 mg/dl 3.BUN: 8.2 mg/dl4.TLC: 33,500/cu.mm 5.Hemoglobin: 12.1 g/dl6.Platelets: 377,000/cu.mm | 1.Na+: 139 mEq/L 2.K+: 5.4 mEq/L 3.Total bilirubin: 1.3 mg/dl 4.Direct bilirubin: 0.9 mg/dl 5.SGPT: 20 IU/L 6.SGOT: 32 IU/L |
Case 2
38 years old female came to ER with chief complaint of: Abdominal pain X 8 hours
Vitals:
| Pulse rate | BP | RR | TPR | SpO2 | Triage Level |
| 76/min | 110/70 mmHg | 22/min | 98 F | 98% in RA | III |
Abdominal pain since 2 days – was intermittent but constant and more severe since last 8 hours. Pain was on Epigastric region and Left iliac fossa. There was a h/o 1 episode of non-bilious vomiting in the morning. There was a h/o decreased urinary frequency and passage of dark colored urine. Bowel habit was normal.
LMP: 2072/05/29
G/C: Agonized; Lying still on bed
Pallor: Present; Icterus: Absent
P/A:
- Guarding present
- Tenderness Diffuse but more marked on Epigastric region and Left Iliac fossa
- Rebound tenderness and Epigastric region and Left Iliac fossa
- Bowel sound: Present
Chest, CVS, CNS: NAD
Provisional diagnosis: Acute abdomen – to rule out hollow viscus perforation
Differential diagnoses:
- Acute Pancreatitis
- Acute Cholecystitis
- Inferior wall MI
- Peptic Ulcer Disease
Lab Reports:
- Serum Amylase: 2,399 IU/L
- TLC: 14,100/cu.mm
- DLC: Neutrophils 87%; Lymphocytes 12%; Eosinophil 1%
- Platelets: 3,05,000/cu.mm
- Urine R/E: All parameters within normal limits
- UPT: Negative
- Serum Calcium: 8.1 mg/dl
- LFTs: Total Bilirubin 2.1 mg/dl; Direct Bilirubin: 1.3 mg/dl; ALT: 117 U/L; AST 219 U/L; ALP 307 U/L
Imaging:
Abdominal X-ray showing both domes of diaphragms:
- No gas under diaphragm (perforation ruled out)
- No significant abnormalities detected
USG abdomen:
- Cholelithiasis (max 10 mm) with slightly distended Gall bladder
- Dilated proximal CBD (max 12 mm); Mild IHBD dilation
- Linear calcification in segment VIII of liver
- Bilateral minimal pleural effusion
Diagnosis: Acute Mild Biliary Pancreatitis with Cholelithiasis with ? Choledocholithiasis
Management at ER:
- IV cannulation done
- Patient kept NPO
- Inj. Tramadol 50 mg IV Stat
- Inj. Ondem 4 mg IV Stat
- Inj. Pantoprazole 40 mg IV Stat
- Inj. Pethidine 50 mg and Inj. Phenargan 25 mg IM Stat (After 1 hour)
- Inj. NS 1 Pint over 1 hour
- Inj. NS 1 Pint over 2 hours
Disposition from ER:
Admitted to Surgical ward after consultation with following advices:
- Counselling for probability of requirement of ICU support
- NPO and Foley catheterization
- Inj. NS 3 Pint and Inj. 5% DW 3 Pint over 24 hours
- Inj. Ceftriaxone 1 gm IV BD and Inj. Metronidazole 500 mg IV BD
- Inj. Pethidine 50 mg + Phenargan 25 mg IM TID
- Inj. Pantoprazole 40 mg IV BD
- Monitoring of Urine output
After Admission to Surgical Ward:
Added medications:
- 3 doses of Vitamin K
- Ketorolac instead of Pethidine
Lab Reports after few days:
- Amylase: 1408 IU/L
- Calcium: 8.3 mg/dl
- Total Bilirubin: 2 mg/dl; Direct Bilirubin: 1.1 mg/dl; AST: 77 IU/L; ALT 74 IU/L; ALP 191 IU/L
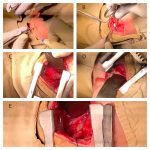
Cholecystectomy done:
- Due to poor visualization of the Calot’s triangle, Laparscopic cholecystectomy was converted into acute cholecystectomy
- OT findings:
- Gall bladder distended
- Common Bile Duct dilated
- 2 stones – faceted 2X2 cm size
Definition of Acute Pancreatitis
Reversible process characterized by:
- Interstitial edema
- Infiltration by acute inflammatory cells
- Necrosis, apoptosis, and hemorrhage
Diagnosis of Acute Pancreatitis
Requires at least 2 of 3 of the following criteria:
- Acute Pancreatitis suggestive abdominal pain
- Serum amylase and/or lipase activity ≥ 3 X the upper limit of normal
- Acute Pancreatitis suggestive/compatible imaging
Diagnosis of Acute Recurrent Pancreatitis
Requires at least two distinct episodes of AP along with:
- Complete resolution of pain (≥1-month pain-free interval between the diagnoses of Acute Pancreatitis) OR
- Complete normalization of serum pancreatic enzyme levels (amylase and lipase), before the subsequent episode of Acute Pancreatitis is diagnosed, along with complete resolution of pain symptoms, irrespective of a specific time interval between Acute Pancreatitis episodes
Etiology of Acute Pancreatitis
Mnemonic: GET SMASHED
- G: Gall stones *
- E: Ethanol *
- T: Trauma *
- S: Surgical (Post-operative*), Scorpion sting (Tityus trinitatis)
- M: Mumps and Coxsackie infection, Mysterious (Idiopathic *)
- A: Autoimmune (Polyarteritis Nodosa)
- S: SPINK-1 (Serine Protease Inhibitor Kajal Type 1), PRSS1 mutation (cationic trypsinogen) i.e. Genetic
- H: Hypertriglyceridemia *, Hypercalcemia, Hypothermia
- E: ERCP *
- D: Drugs *(Corticosteroids, Thiazides, Valproate, Azathioprine, Estrogen, Sulfonamides, Tetracycline, 6-Mercaptopurine, anti-HIV medications)
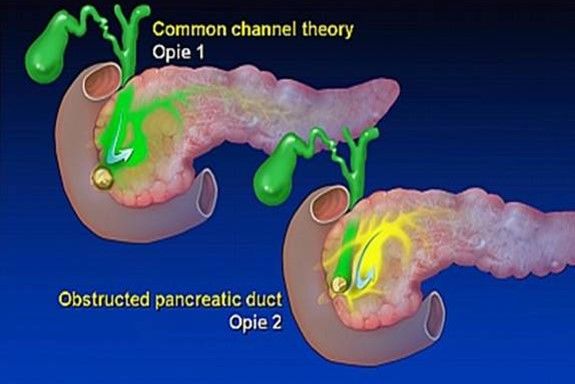
How gallstones causes pancreatitis?
Theory 1: Reflux of bile into the pancreas due to obstruction at Ampulla of Vater
Theory 2: Intraductal hypertension due to outflow blockade at pancreatic duct and penetration of secretion into interstitial tissue
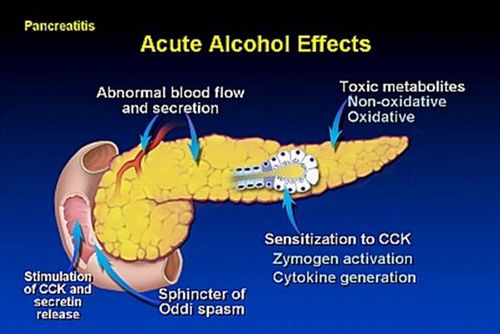
How alcohol causes pancreatitis?
Recommendations for Etiological Diagnosis:
| 1. | Transabdominal USG should be performed in all patients with acute pancreatitis |
| 2. | In the absence of gallstones and/or history of significant history of alcohol use, a serum triglyceride should be obtained and considered the etiology if >1,000 mg/dl |
| 3. | In a patient > 40 years, a pancreatic tumor should be considered as a possible cause |
| 4. | Endoscopic investigation in patients with acute idiopathic pancreatitis should be limited |
| 5. | Patients with idiopathic pancreatitis should be referred to centers of expertise (if available) |
| 6. | Genetic testing may be considered in young patients (<30 years old) if no cause is evident and a family history of pancreatic disease is present |
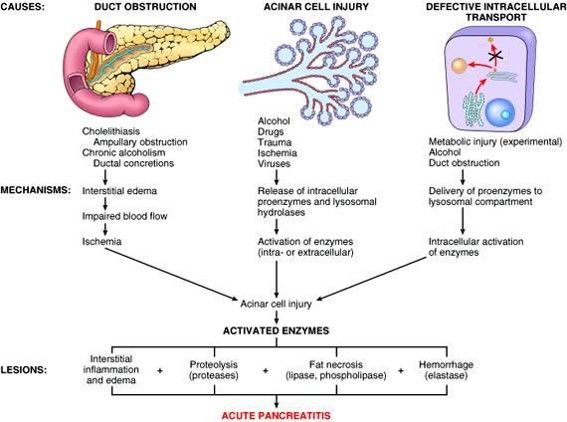
Pathophysiology of Acute Pancreatitis
1st Phase:
Premature activation of trypsin within pancreatic acinar cell leading to activation of varieties of injurious pancreatic enzymes.
2nd Phase:
Intrapancreatic inflammation
3rd Phase:
Extrapancreatic inflammation including ARDS
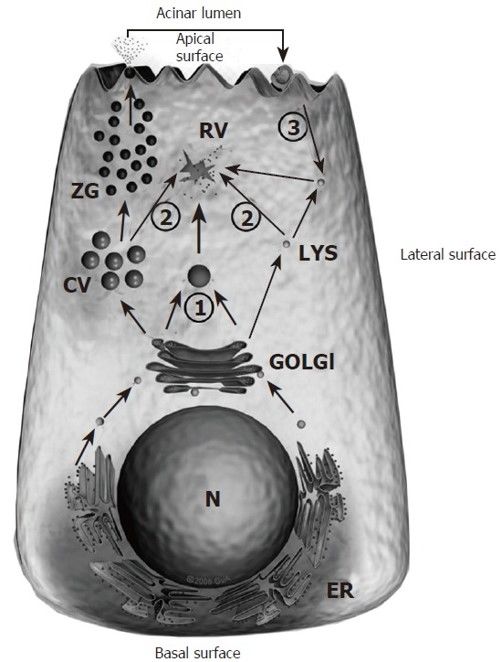
Co-localization theory (Pathogenesis at Cellular level):
- Ductal Obstruction
- Improper co-localization of lysosomal hydrolases (Cathepsin-B) with zymogens (Trypsinogen) into vacuolar structure within pancreatic acinar cell
- Activation of digestive enzyme zymogen (trypsinogen to trypsin)
- Activation of other digestive enzyme zymogens
- Initiation of auto-digestion within pancreas
Symptoms of Acute Pancreatitis
- Location: Upper abdomen/Epigastric
- Length (Duration): Hours to a day
- Intensity: Gradually becomes severe (not relieved by ordinary analgesics)
- Quality: Constant and Dull
- Onset: Sudden
- Radiation: Back
- Associated symptoms: Nausea, Vomiting, Anorexia, Abdominal distension
- Aggravating factors: Eating or drinking (specially alcohol)
- Alleviating factors: Leaning forward, Curl up (Fetal position)
Signs of Acute Pancreatitis
General condition: Distressed, Anxious
Vitals: Fever, Tachycardia, Hypotension, Tachypnea
Cardinals: Jaundice, Cyanosis, Dehydration
Respiratory: Signs of pleural effusion – usually left sided (sometimes)
Abdominal: Marked epigastric tenderness with voluntary and involuntary guarding +/- rigidity, Abdominal distension, Reduced bowel sound, Palpable pseudocyst (sometimes)
Uncommon signs associated with severe necrotizing pancreatitis:
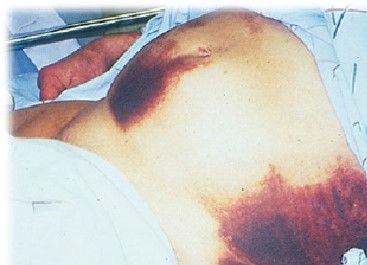
- Cullen’s sign (Periumbilical discoloration due to peritoneal hemorrhage)
- Grey-Turner’s sign (Flank discoloration due to retroperitoneal hemorrhage)
- Fox’s sign (Discoloration below inguinal ligament or at the base of penis)
- Erythematous skin nodules (Subcutaneous fat necrosis)
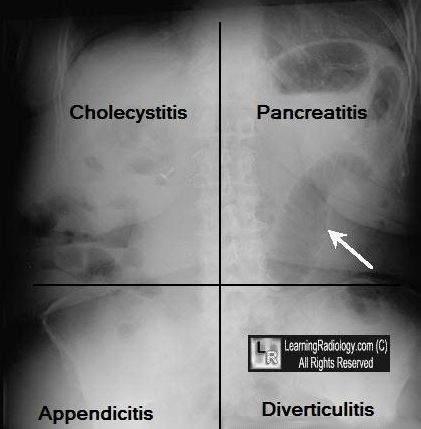
Differential diagnosis of Acute Epigastric Pain
| Cholangitis | Charcot’s triad – RUQ pain, fever, jaundice; Bilirubin > 4; AST > 1000 |
| Acute cholecystitis & biliary colic | Pain epigastrium and RUQ, radiates to right shoulder or shoulder blade, LFT’s increased |
| Pancreatitis | Pain constant & gradually increases over 30- 60 minutes; pain radiates to the back; Vomiting & increased amylase; LFT’s may be increased if due to gall stones. H/O and P/E correlate – severe pain associated with marked tenderness |
| Intestinal obstruction | Pain = colicky, obstructive pattern seen on imaging |
| Dissecting aortic aneurysm | Sudden onset, pain may radiate to lower extremities |
| Perforated PUD | RUQ or mid-epigastric pain, sudden onset, free intraperitoneal air, perforation of PUD mimics cholecystitis |
| Pulmonary/pleural disease | Consider if pleuritic pain is a dominant symptom |
| Hepatitis | Malaise, ALT > 1000 |
| Inferior wall MI | Mid-epigastric pain, SOB, abnormal ECG; MI should be in the differential diagnosis in all patients with upper abdominal pain |
| Mesenteric ischemia | Abdominal pain severe, out of proportion to tenderness with a fairly benign exam. Look for post-prandial abdominal pain, weight loss and abdominal bruit. |
| Fitz-Hugh-Curtis syndrome | Gonococcal perihepatitis w/ RUQ pain, adnexal tenderness |
| Pneumonia | Fever and respiratory symptomss (dyspnea, cough, sputum, chest pain) present |
| Appendicitis | Pain may start in epigastrium but eventually moves to RLQ test |
Some other eponymous signs
1. Korte’s sign: painful resistance at a lumbar bar in a epigastric area on 6–7 cm above umbilicus (location of head of pancreas)
2. Voskresynskyy’s sign: absence of pulsation of abdominal aorta in an epigastric area
3. Mayo-Robson’s sign: left consto-vertebral angle (CVA) tenderness
4. Rozdolskyy’s sign: percussion tenderness above pancreas
Frequency of signs and symptoms in Acute Pancreatitis
- Abdominal pain: 95–100%
- Epigastric tenderness: 95–100%
- Nausea and vomiting: 70–90%
- Low-grade fever: 70–85%
- Hypotension: 20–40%
- Jaundice: 30%
- Grey Turner/Cullen sign: <5%
Complications of Acute Pancreatitis
Local complications of Acute Pancreatitis
Necrosis:
- Sterile
- Infected
Pancreatic necrosis is defined as diffuse or focal areas of nonviable pancreatic parenchyma > 3 cm in size or > 30% of the pancreas
Pancreatic fluid collections:
- Pancreatic abscess
- Pancreatic pseudocyst (Pain, rupture, hemorrhage, infection, obstruction of GIT)
- Pancreatic pseudoaneurysm (Erosion of adjacent artery by pseudocyst)
Pancreatic ascites
- Disruption of main pancreatic duct
- Leaking pseudocyst
Involvement of contiguous organs by necrotizing pancreatitis
- Massive intraperitoneal hemorrhage
- Thrombosis of blood vessels (splenic vein i.e. sinistral portal HTN, portal vein)
- Bowel obstruction
Obstructive Jaundice
Systemic Complications of Acute Pancreatitis
Pulmonary: Pleural effusion, Atelectasis, Mediastinal abscess, Pneumonitis, ARDS
Cardiovascular: Hypotension (Hypovolemia), Sudden death, Pericardial effusion
Hematologic: Disseminated Intravascular Coagulation (DIC)
Gastrointestinal hemorrhage: Peptic ulcer disease, Erosive gastritis, Hemorrhagic pancreatic necrosis with erosion into major blood vessels, Portal vein thrombosis (Variceal hemorrhages)
Renal: Oliguria, Azotemia, Renal artery/vein thrombosis, Acute Tubular Necrosis
Metabolic: Hyperglycemia, Hypocalcemia, Encephalopathy, Sudden blindness (Purtscher’s retinopathy)
CNS: Psychosis, Fat emboli
Timeline of complications in Acute Pancreatitis
Period 1 (Hemodynamic violations and pancreatogenic shock): Initial 2-3 days
Period 2 (Insufficiency of parenchymatous organs i.e. MODS): 3rd-7th days
Period 3 (Postnecrosis dystrophic and festering complications): 1-2 weeks after disease onset i.e. Regenerative changes begin in the background of necrosis
- Parapancreatic infiltrate and cysts
- Cystic fibrosis of pancreas
- Aseptic retroperitoneal phlegmon
- Infection of pancreatic necrosis
- Erosive bleeding
- External and internal fistula
Lab Studies in Acute Pancreatitis
1. Serum amylase and lipase: ↑ by 3X of upper-limit is diagnostic
2. LFTs:
- ↑ ALT and AST (ALT ↑ by around 3X i.e. >150 IU/L is diagnostic of gall stone pancreatitis)
- ↑ALP, ↑bilirubin, ↓albumin
3. RFTs: BUN and Creatinine (to rule out renal failure)
4. CBC and HCt:
- Leukocytosis with shift to left (Inflammation or SIRS)
- ↑HCt (Hemoconcentration due to fluid sequestration)
- ↓HCt (Dehydration or Hemorrhage)
5. Blood biochemistry:
- Blood sugar: may ↑ due to insulin producing Beta-cell dysfunction
- Serum calcium: ↓ (due to hypoalbuminemia or fat necrosis) or ↑ (if hypercalcemia is etiology)
- Lipid profile: to rule out hypertriglyceridemia as the cause
6. ABG: every 12 hrs for 1st 3 days (to monitor oxygenation and acid-base status)
7. Other: CRP, Trypsin, Trypsinogen-2, LDH, Phospholipase A
Lipase is more specific than amylase (hence preferred). Lipase rises within 4-8 hours of pain onset. Amylase rises within 6 hours of pain onset. Amylase may be normal during acute inflammation due to significant pancreatic destruction. Amylase normalizes in blood by 3-5 days due to increased excretion in urine. Lipase remains elevated for 7-10 days.
Other causes of ↑ amylase: Macroamylasemia, Renal failure, Mumps parotitis, ERCP induced, Esophageal perforation, Pregnancy
Imaging Studies in Acute Pancreatitis
Plain Chest X-Ray: Pleural effusion, ARDS (Atelectasis and Basal infiltrates), Elevation of left diaphragm
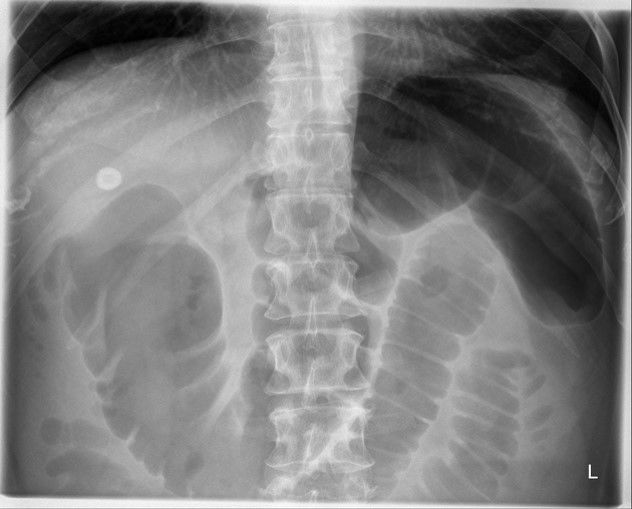
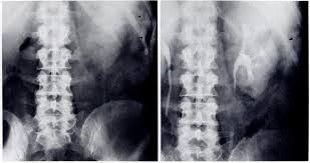
Plain Abdomina X-Ray (erect):
- To rule out perforated peptic ulcer
- Sentinel loop sign, Colon ‘cut-off’ sign, Renal halo sign
- May show gallstone, pancreatic calcification
Abdominal USG: Can detect gallstones, biliary obstruction, pseudocyst formation
CT abdomen: may be required if diagnosis uncertain, to rule out and find degree of: Peripancreatic collection; Necrosis and Abscess
CT Guidelines for Acute Pancreatitis
1. In the acute setting (<48–72 hours), CECT should not be performed when a typical clinical presentation and unequivocal elevations of amylase and lipase are present.
2. In the acute setting, CECT should be performed if the clinical presentation and amylase and lipase levels are equivocal.
3. Early (within the first 72 hours) imaging with CT may underestimate the full severity of the disease.
4. CECT after 48–72 hours will detect pancreatic and peripancreatic necrosis as well as acute pancreatic fluid collections.
5. Delayed CECT (>7–21 days after the onset of symptoms) is very effective in assessing severity and will guide management, including image-guided aspiration and/or drainage as well as other forms of minimally invasive drainage.
6. CECT should be performed when there is a significant deterioration of the patient’s condition, including an acute drop in hemoglobin and hematocrit, tachycardia, and hypotension, an abrupt change in fever, or leukocytosis.
Colon Cut-off sign
- Distended proximal colon + Splenic flexure narrowing + Collapse of descending colon starting from region of pancreatic inflammation
- Extra-pancreatic extension of inflammation into phrenico-colic ligament resulting in splenic flexure spasm
- D/D: IBD, Ca. colon, Mesenteric ischemia
Sentinel Loop sign
- Localized isolated ileus seen near the site of injured viscus or inflamed organ
- Body’s effort to localize traumatic or inflamed lesions
- Localized paralysis followed by gas accumulation
- Sentinel = A soldier stationed as a guard to challenge all comers and prevent a surprise attack
- Site helpful in diagnosis
Renal Halo sign
- Peripancreatic inflammation extension into pararenal space
- Radiolucent halo of inflammation in anterior pararenal space contrasts with perirenal fat; more common on left side
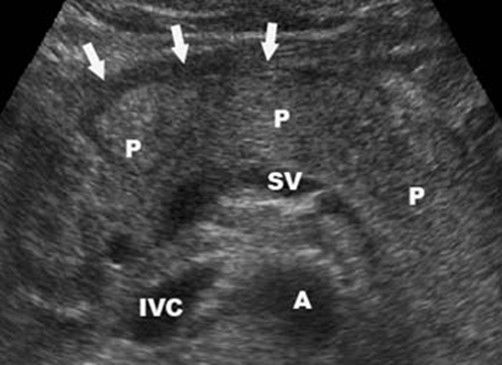
Acute Pancreatitis in Ultrasonography (USG)
- Pancreatic gland (P) is edematous and there is a fluid visible in front of the pancreas (Black anechogenic strip marked by arrows).
- Other anatomical structures we see splenic vein (SV), aorta (A) and inferior vena cava (IVC).
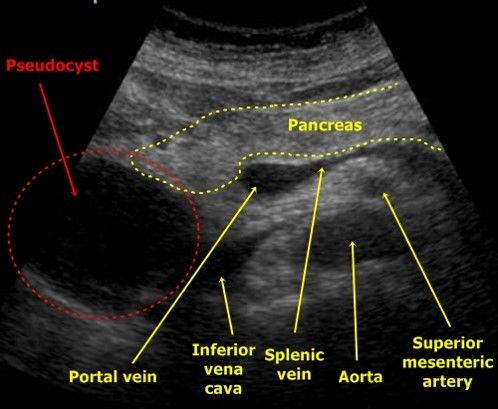
Pancreatic Pseudocyst in USG
- Regular anechogenic mass in contact to the head of pancreas
- A pancreatic tumor may look similar but is not as anechogenic as a pseudocyst
SCORINGS FOR ACUTE PANCREATITIS
Modified CT Score Index (CTSI) for Acute Pancreatitis
A) Pancreatic inflammation
0: normal pancreas
2: intrinsic pancreatic abnormalities with or without inflammatory changes in peripancreatic fat
4: pancreatic or peripancreatic fluid collection or peripancreatic fat necrosis
B) Pancreatic necrosis
0: none
2: 30% or less
4: more than 30%
C) Extrapancreatic complications
2: one or more of pleural effusion, ascites, vascular complications, parenchymal complications and or gastrointestinal involvement
INTERPRETATION:
Total points out of 10 to grade pancreatitis:
- 0-3: mild
- 4-7: moderate
- 8-10: severe
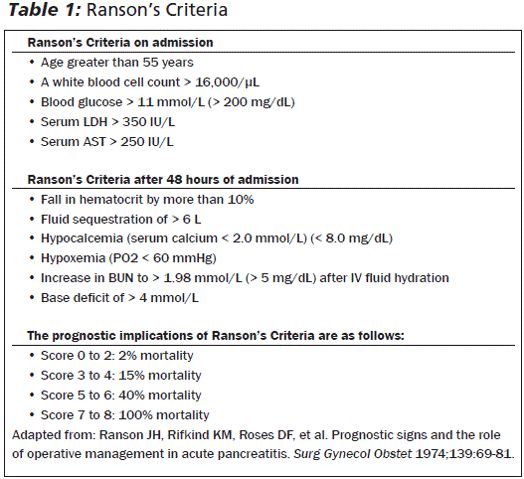
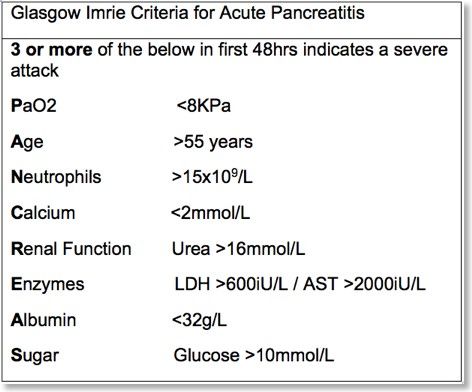
Ranson’s score
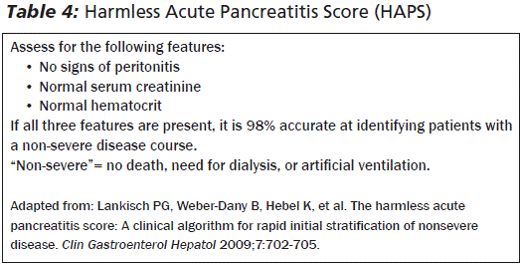
HAPS score
Modified Glasgow Score
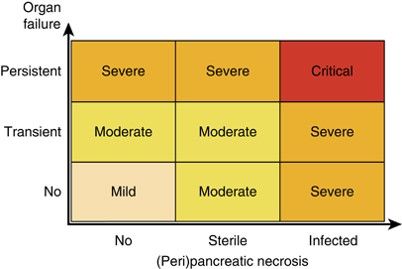
Atlanta Score for Acute Pancreatitis
| Atlanta criteria (1993) | Atlanta Revision (2013) |
| Mild acute pancreatitis | Mild acute pancreatitis |
| Absence of organ failure | Absence of organ failure |
| Absence of local complications | Absence of local complications |
| Severe acute pancreatitis | Moderately severe acute pancreatitis |
| 1.Local complications AND/OR | 1.Local complications AND/OR |
| 2.Organ failure | 2.Transient organ failure (< 48 h) |
| GI bleeding (> 500 cc/24 hr) | Severe acute pancreatitis |
| Shock – SBP ≤ 90 mm Hg | Persistent organ failure > 48 hr |
| PaO 2 ≤ 60% | |
| Creatinine ≥ 2 mg/dl | |
| GI, gastrointestinal; SBP, systolic blood pressure. Persistent organ failure is now defined by a Modified Marshal Score | |
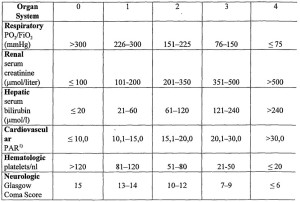
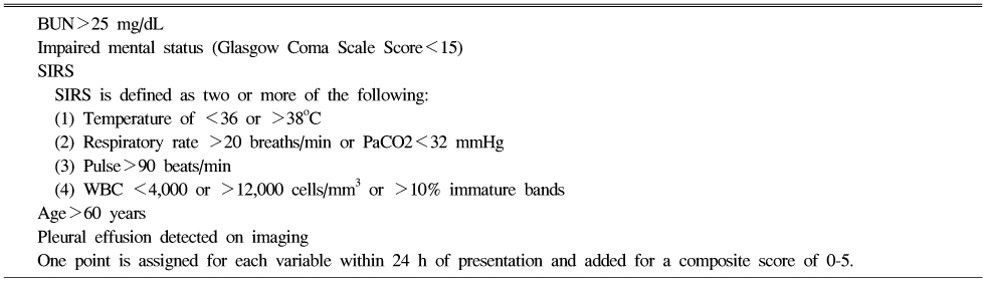
BISAP score (Bedside Index for Severity in Acute Pancreatitis)
Patients with a BISAP Score >0 had an increasing risk of mortality, with mortality increasing significantly with a score of 3 or greater. A score of 5 had a mortality rate of 22%.
Findings associated with Severe course
| Patient characteristics |
| Age >55 years |
| Obesity (BMI >30 kg/m2) |
| Altered mental status |
| Comorbid disease |
| The systemic inflammatory response syndrome (SIRS) Presence of >2 of the following criteria: |
| – pulse >90 beats/min |
| – respirations >20/min or PaCO2 >32 mm Hg |
| – temperature >38 °C or <36 °C |
| – WBC count >12,000 or <4,000 cells/mm3 or >10% immature neutrophils (bands) |
| Laboratory findings |
| BUN >20 mg/dl |
| Rising BUN |
| HCT >44% |
| Rising HCT |
| Elevated creatinine |
| Radiology findings |
| Pleural effusions |
| Pulmonary infiltrates |
| Multiple or extensive extrapancreatic collections |
Others:
- DIC
- Platelets ≤100,000/mm3
- Fibrinogen ≤100 mg/dL
- Fibrin split products >80μg/mL
- Severe metabolic disturbance (serum calcium ≤7.5 mg/dL)
- CRP > 150 mg/L within 1st 72 hours
How BMI plays a role in severity of acute pancreatitis?
Adipose tissue or fat releases a large amount of inflammatory cytokines, what we call adipokines, 50% of the amount of TNF-a and MCP-1 is released by adipocyte tissue, but also they are fat specific cytokines such as resistant with fat and adiponectin.
Management of Acute Pancreatitis
A) Initial assessment and Risk Stratification
| 1. | Hemodynamic status should be assessed immediately upon presentation and resuscitative measures begun as needed |
| 2. | Risk assessment should be performed to stratify patients into higher- and lower-risk categories to assist triage, such as admission to an intensive care setting |
| 3. | Patients with organ failure should be admitted to an intensive care unit or intermediary care setting whenever possible |
- Airway (Patency + Vomitus obstructing airway? )
- Breathing (Respiratory depth and rate + SpO2 + Wheeze/crackles?)
- Circulation (CRT + Pulse + BP + Mucous membrane)
- Disability (GCS + Glucose levels)
- Exposure:
- Examine abdomen
- Cullen’s and Grey-Turner’s sign?
- Tenderness on palpation?
- Any signs of peritonism – rebound, guarding or percussion tenderness?
- Flank tenderness?
- Temperature measurement
B) Airway and Breathing support
- Maintatin SpO2 > 94%
- High flow oxygen with non-rebreather mask
- Pulmonary complaints necessitate supplemental oxygen
- Endotracheal intubation for ARDS or severe encephalopathy
C) Fluid resuscitation in Acute Pancreatitis
- Aggressive hydration: 250-500 ml per hour of isotonic crystalloid solution
- Early aggressive intravenous hydration is most beneficial the first 12–24 h, and may have little benefit beyond
- In severe volume depletion (hypotension and tachycardia) bolus may be needed
- RL may be the preferred isotonic crystalloid replacement fluid
- Fluid requirements should be reassessed at frequent intervals within 6 h of admission and for the next 24–48 h.
- The goal of aggressive hydration should be to decrease the BUN.
D) Blood products in Acute Pancreatitis:
- In hemorrhagic pancreatitis, transfuse to hematocrit level of 30%.
- Fresh-frozen plasma and platelets if coagulopathic and bleeding.
E) Correct Electrolyte Abnormalities if present:
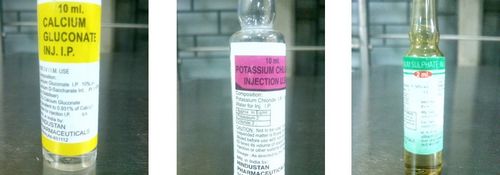
- Hypocalcemia: Calcium gluconate 10%: 10 mL IV over 15–20 min
- Hypokalemia: KCl 10 mEq/h IV over 1 hr
- Hypomagnesemia (alcohol abuse): MgSO4: 2 g IV piggyback
F) Pain Management in Acute Pancreatitis
- Past – Morphine blamed to cause spasms of the sphincter of Oddi (Pethidine was the analgesic of choice)
- Recently studies showed that morphine has no proven significantly unfavorable influence on the course of AP
- Metamizole (2g/8h i.v.) vs morphine (10mg/4h s.c.): metamizole resulted in more frequent and quicker pain relief
- Buprenorphine vs Pethidine: longer-acting with similar analgesic capacity as pethidine, but a lower potential to cause physical opioid dependence
- Buprenorphine vs procaine continuous intravenous (i.v.) infusion: Patient on procaine likely to demand additional analgesics and had relatively lesser pain relief
- Procaine (2g/24hours as continuous i.v. infusion) vs pentazocine (bolus i.v. every 6 hours): Patient on procaine more likely to demand additional analgesics (98% versus 44%)
G) Antiemetics in Acute Pancreatitis
- Ondansetron 4 mg IV stat
H) Nasogastric suctioning in Acute Pancreatitis
- Not useful in cases of mild pancreatitis
- Beneficial in severe pancreatitis, intractable vomiting, severe ileus and severe abdominal distension
I) Antibiotics in Acute Pancreatitis
- Given for an extrapancreatic infection, such as cholangitis, catheter-acquired infections, bacteremia, urinary tract infections, pneumonia
- Routine use of prophylactic antibiotics in patients with severe acute pancreatitis is not recommended
- Use of antibiotics in patients with sterile necrosis to prevent the development of infected necrosis is not recommended
- Infected necrosis should be considered in patients with pancreatic or extrapancreatic necrosis who deteriorate or fail to improve after 7–10 days of hospitalization. In these patients, either (i) initial CT-guided fine needle aspiration (FNA) for Gram stain and culture to guide use of appropriate antibiotics or (ii) empiric use of antibiotics without CT FNA
- In patients with infected necrosis, antibiotics known to penetrate pancreatic necrosis, such as carbapenems, quinolones, and metronidazole
J) Nutrition in Acute Pancreatitis
- In mild AP, oral feedings can be started immediately if there is no nausea and vomiting, and abdominal pain has resolved.
- In mild AP, initiation of feeding with a low-fat solid diet appears as safe as a clear liquid diet.
- In severe AP, enteral nutrition is recommended to prevent infectious complications. Parenteral nutrition should be avoided unless the enteral route is not available, not tolerated, or not meeting caloric requirements.
- Nasogastric delivery and nasojejunal delivery of enteral feeding appear comparable in efficacy and safety.
- TPN for specific indications like Paralytic ileus.
K) Specific drug therapy for Acute Pancreatitis
No proven therapy for the treatment of acute pancreatitis. All proved disappointing in large randomized studies:
- Antiproteases: Gabexate
- Antisecretory agents: Octreotide and Somatostatin
- Anti-inflammatory agents: Lexipafant
Disposition from Emergency Department
Admission Criteria
- Acute pancreatitis with significant pain, nausea, vomiting
- ICU admission for hemorrhagic/necrotizing pancreatitis
Discharge Criteria
- Mild acute pancreatitis without evidence of biliary tract disease and able to tolerate oral fluids
- Chronic pancreatitis with minimal abdominal pain and able to tolerate oral fluids
Issues for Referral
- Surgical/GI consultation for ERCP in severe pancreatitis with cholangitis or biliary obstruction
- Emergent surgical consultation mandatory in cases of suspected ruptured pseudocyst or pseudocyst hemorrhage, as definitive treatment is emergent laparotomy
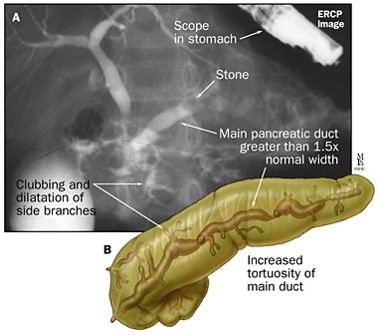
ERCP in Acute Pancreatitis
- Patients with acute pancreatitis and concurrent acute cholangitis should undergo ERCP within 24 h of admission.
- ERCP is not needed in most patients with gallstone pancreatitis who lack laboratory or clinical evidence of ongoing biliary obstruction.
- In the absence of cholangitis and/or jaundice, MRCP or endoscopic ultrasound (EUS) rather than diagnostic ERCP should be used to screen for choledocholithiasis if highly suspected.
- Pancreatic duct stents and/or postprocedure rectal nonsteroidal anti-inflammatory drug (NSAID) suppositories should be utilized to prevent severe post-ERCP pancreatitis in high-risk patients.
Surgery in Acute Pancreatitis (AP)
- In patients with mild AP, found to have gallstones in the gallbladder, a cholecystectomy should be performed before discharge to prevent a recurrence of AP
- In a patient with necrotizing biliary AP, in order to prevent infection, cholecystectomy is to be deferred until active inflammation subsides and fluid collections resolve or stabilize
- Asymptomatic pseudocysts and pancreatic and/or extrapancreatic necrosis do not warrant intervention regardless of size, location, and/or extension
- In stable patients with infected necrosis, surgical, radiologic, and/or endoscopic drainage should be delayed preferably for more than 4 weeks to allow liquefication of the contents and the development of a fibrous wall around the necrosis (walled-off necrosis)
- In symptomatic patients with infected necrosis, minimally invasive methods of necrosectomy are preferred to open necrosectomy.

He is the section editor of Orthopedics in Epomedicine. He searches for and share simpler ways to make complicated medical topics simple. He also loves writing poetry, listening and playing music.

Thank you this was very helpful!
this is perfect. thanks a lot
This was really very helpful. Thank you Dr. Shrestha!