Structure of Heme
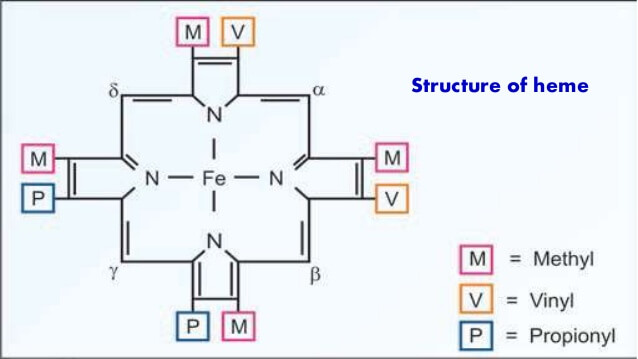
Heme is a ferro-proto-porphyrin.
Heme = Protoporphyrin IX ring + Iron (Fe) in center
Protoporphyrin IX = porphyrin with attachment to 4 Methyl, 2 Propionyl and 2 Vinyl groups.
Porphyrin = Cyclic structure with 4 pyrrole rings
Pyrrole rings are derived from Porphobilinogen (PBG)
Hydroxymethylbilane = Linear structure with 4 pyrrole rings
Uroporphyrobilinogen = Cyclization of hydroxymethylbilane
Steps in Heme Synthesis
Step 1: We need a Pyrrole ring
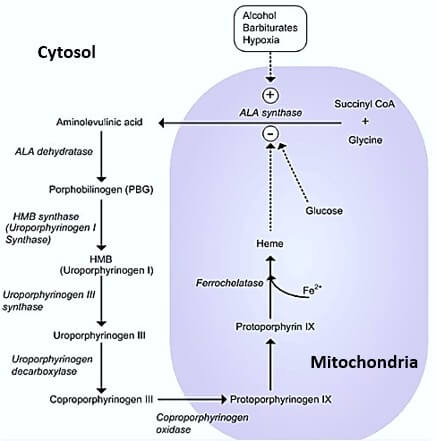
1. Glycine + Succinyl-CoA = δ-ALA (Amino-Levulinic Acid)
- Catalyzed by ALA synthase
- Requires Pyridoxal-phosphate (Vit. B6) for activation of Glycine
- Rate-limiting enzyme (Repressed by heme)
- Occurs in mitochondria
2. ALA leaves mitochondria into the cytosol
3. ALA + ALA or 2 X ALA = Porphobilinogen
- Condensed by ALA dehydratase
- Inhibited by Lead (Pb)
We now have a pyrrole ring.
Step 2: We need 4 Pyrrole rings
4. 4 X Porphobilinogen = Hydroxymethylbilane or Pre-uroporphyrinogen
- By Porphobilinogen Deaminase aka Hydroxymethylbilane synthase
- Hydroxymethylbilane is the 1st porphyrin of the pathway (absorbs light and cause photosensitivity)
Now, we have 4 pyrrole rings arranged linearly.
Step 3: We need a Porphyrin ring
5. Hydroxymethylbilane = Uroporphyrinogen III
- Cyclization or ring closure by Uroporphyrinogen III synthase
Step 4: We need Proto-porphyrin IX
6. Uroporphyrinogen III = Coproporphyrinogen III
- Ring modification (removal of 4 CO2) by Uroporphyrinogen Decarboxylase
7. Coproporphyrinogen III leaves cytosol and enters back into mitochondria
8. Coproporphyrinogen III = Proto-porphyrin IX
- By oxidation reactions
Step 5: We need Heme
Since, heme is a ferro-protoporphyrin – we need to add an iron on the center of the protoporphyrin ring.
9. Proto-porphyrin IX + Fe²+ = Heme
- By Ferrochelatase
- Inhibited by lead (Pb)
We have found the Heme !!! Mission accomplished.
Location of Heme Synthesis
Both the mitochondria and cytosol:
- ALA leaves mitochondria and Copro-porphyrinogen III enters back into the mitochondria
So, can the RBCs synthesize Heme?
No. RBCs lack mitochondria. So, heme is synthesized in the RBC progenitor cells.
Is Heme synthesized only in RBC precursors?
No. Heme is synthesized in almost every cells because heme is not only the part of hemoglobin but other important molecules as well. These will be discussed below.
Heme as a Component of Important Structures
- Hemoglobin
- Myoglobin
- Cytochromes
- Enzymes:
- Catalase
- Peroxidase
- Guanylate cyclase (stimulated by nitric oxide)
Defects in Heme Synthesis
Porphyria in General
As discussed earlier, Hydroxymethylbilane is the 1st porphyrin of the pathway which is able to absorb light and cause photosensitivity. Hence, defects above will have “No Photosensitivity” and defects below will have “Photosensitivity“.
Clinical features depend upon the accumulation of substrates:
Block early in the pathway: Accumulation of ALA
ALA is responsible for neurological symptoms:
- Abdominal pain
- Neuropsychiatric symptoms
- Motor: Muscle weakness
- Autonomic: Vomiting, Constipation
Mechanism of ALA toxicity:
- Inhibition of ATPase in nervous tissue by ALA
- ALA may be taken up by brain which causes conduction paralysis
Block later in the pathway: Accumulation of porphyrinogens
Porphyrinogen accumulation is responsible for photosensitivity.
Mechanism of photosensitivity:
- Porphyrinogens absorbs light and auto-oxidizes to corresponding Porphyrin derivatives.
- These porphyrins react with molecular oxygen to form oxygen radicals in the skin.
- Oxygen radicals damage lysososmes which further releases degradative enzymes.
- This leads to skin damage.
Exacerbated by P450 inducing drugs:
- Cytochrome P450 induction = Increased synthesis of Cytochrome P450 (has heme as a component) = Decreased heme levels = Decreased repression of ALA synthase = Increased porphyrin precursor synthesis
- Examples:
- Barbiturates
- Alcohol
- Cigarette smoking
- Anticonvulsants
- Horomones
Fasting-induced porphyria and Role of glucose infusion:
Fasting increases the level of a protein, proliferator-activated receptor γ coactivator 1α (PGC-1α), which is involved in creating glucose in the liver. They also found that this protein regulates a key enzyme in the heme biosynthesis pathway, 5-aminolevulinate synthase (ALAS-1). Increased levels of PGC-1α resulted in increased production of ALAS-1. This in turn led to a build-up of precursor heme molecules, which, caused acute attacks of porphyria. 1
Photo-sensitivity may be benefited by beta-carotene: Anti-oxidant
Inheritance Pattern of porphyria:
Porphyria is caused as a result of diminished feedback inhibition by end-product due to enzyme deficiencies. Hence, these are autosomal dominant disorders with exception to Congenital Erythropoietic Porphyria which is autosomal recessive.
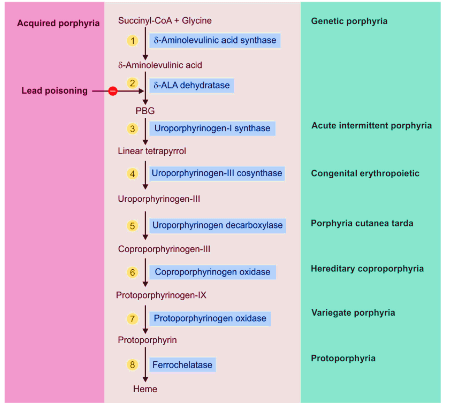
Lead toxicity can causes Acquired form of Porphyria by inhibiting 2 enzymes:
- ALA dehydratase
- Ferrochelatase
Types of Porphyria according to Enzyme Defects:
- ALA synthase: X-linked Porphyria
- ALA dehydratase: Inhibited in Lead poisoning
- Porphobilinogen deaminase or Hydroxymethylbilane synthase: Acute Intermittent Porphyria
- Uroporphyrinogen III synthase: Congenital erythropoietic porphyria
- Uroporphyrinogen decarboxylase: Porphyria cutanea tarda; Also inhibited by hexachlorobenzene (BHC)
- Coproporphyrinogen oxidase: Coproporphyria
- Protoporphyrinogen oxidase: Variegate porphyria
- Ferrochelatase: Protoporphyria; Also inhibited in lead poisoning
Hepatic Vs Erythropoietic porphyria: Based on the site where heme synthesis is active –
Erythropoietic porphyrias (Bone marrow):
- Congenital erythropoietic porphyria
- Protoporphyria
Hepatic porphyrias (Liver): Others
Porphyria Cutanea Tarda:
- Defect in uroporphyrinogen decarboxylase
- Autosomal dominant
- Most common porphyria
- Late onset (4th or 5th decade)
- Exacerbated by hepatotoxic susbstances as it is a heaptic form of porphyria:
- Alcohol
- Iron
- Accumulation of uroporphyrin:
- Photosensitivity
- Hyperpigementation (Body’s attempt to protect the skin)
- Characteristic red-wine urine
Acute Intermittent Porphyria:
- Defect in Porphobilinogen Deaminase
- Autosomal dominant
- Late onset
- No porphyrin – No photosensitivity
- Accumulation of ALA:
- Abdominal pain
- Episodic psychological symptoms – anxiety, confusion, paranoia, depression
- Exacerbated by barbiturates
- Dark red/brown colored urine: ALA and PBG in urine
Mnemonic
All Congenital Porphyria Comprise Variable Presentation
Remember the pathway and correlate with the mnemonic sequentially.
- All = Acute Intermittent Porphyria (PB deaminase)
- Congenital = Congenital Erythropoietic Porphyria (Uroporphyrinogen III synthase)
- Porphyria = Porphyria Cutanea Tarda (Uroporphyrinogen decarboxylase)
- Comprise = Coproporphyria (Coproporphyrinogen oxidase)
- Variable = Variegate Porphyria (Protoporphyrinogen oxidase)
- Presentation = Protoporphyria (Ferrochelatase)
Hemin
Occurs when Fe³+ is incorporated in proto-porphyrin instead of Fe²+
Used in treatment of acute attacks of porphyria particularly Acute Intermittent Porphyria
Mechanism of Action:
Reduce incorporation of glycine into cellular heme, significantly inhibited formation of protoporphyrin IX and total delta-aminolevulinate. 2
Antimalarial action:
- Malarial parasites are rich in hemin; artemisinin’s reactivity toward hemin may explain its selective toxicity to malarial parasites. 3
- Hemin-chloroquine complex is toxic to parasites. 4
Lead Poisoning
ALA dehydratase and Ferrochelatase are Zn²+ dependent metallo-enzymes. Pb2+ replaces the Zn2+ at the active site and inhibits them.
Features:
- Microcytic sideroblastic anemia
- Coarse basophilic stippling of RBCs
- Increase in ALA without increase in PBG (differentiate from other porphyrias)
- Increased free erythrocyte protoporphyrin
- Lead lines in gum, Neuropathy, Abdominal pain, Nephrotoxicity (deposition in PCT)
- Headache, nausea, memory loss
Vitamin B6 deficiency
ALA synthase requires Vit. B6 (pyridoxine)
Explains sideroblastic anemia with Isoniazid therapy
Iron deficiency
Iron is incorporated into protoporphyrin in the last step to form heme.
Deficiency results in microcytic hypochromic anemia
- Cytoplasmic maturation defect while the nuclear maturation is intact

He is the section editor of Orthopedics in Epomedicine. He searches for and share simpler ways to make complicated medical topics simple. He also loves writing poetry, listening and playing music.
Just what i needed. Thank you
Best way explained…easy to understand basic…thank you.
marvellous…!!! easily understandable presentation of mechanisms of action….really helpful!
Really helpful article.. gained a lot of important points.. thanks a lot 😊
very useful and within in a limited content
extremely good
you didn’t leave anything out!
Excellent contents
Excellent presentation of relevant facts! Just what i needed.
very helpful.