Definitions of Related Terms
A) Ischemic Heart Disease (IHD): It is the imbalance between myocardial oxygen supply and demand, resulting in myocardial hypoxia and accumulation of waste metabolites. The components of IHD are:
- Stable Angina
- Acute Coronary Syndrome (ACS)
B) Stable Angina: Pattern of chronic, predictable, transient chest discomfort during exertion or emotional rest and relieves with rest.
C) Acute Coronary Syndrome (ACS):
Unstable angina (UA) | Non-ST-elevation myocardial infarction (NSTEMI) | ST-elevation myocardial infarction (STEMI) | |
| Thrombus size | partially occlusive | partially occlusive | completely occlusive |
| Myocardial necrosis | absent | present in small amount | present in large amount |
| Serum biomarkers (cTnT, cTnI, CK-MB) | absent | present | present |
Mechanisms of Myocardial Ischemia
A) Fixed vessel narrowing:
i. Coronary flow reserve:
- Compensatory vasodilation that smaller, distal resistance vessels are able to achieve
- 4-6 times the resting value
- 95% coronary arterial resistance is accounted for by small intramural vessels that are not visualized during coronary angiography. About 5% resistance arises within conductive epicardial coronary arteries.
Cardiac Syndrome X: Typical symptoms of angina pectoris without evidence of significant CAD in coronary angiogram – thought to be due to inadequate vasodilator coronary flow reserve. The proposed mechanisms are:
1. Endothelial dysfunction (Microvascular angina)
2. Insulin resistance (Metabolic syndrome)
3. Altered cardiac sensitivity
4. Estrogen deficiency
5. Altered autonomic control
ii. Degree of stenosis: In stable angina, coronary flow reserve is inadequate with >70% of cross-sectional area (equivalent to 50% of lumen diameter by angiography) stenosis – predictable.
- Less predictable episodes: Coronary vasoconstriction associated with atherosclerotic disease – coronary flow reserve is inadequate at lesser degree of stenosis.
iii. Myocardial oxygen demand:
- At rest: Coronary flow reserve may be adequate
- With elevated heart rate and force of contraction: Coronary flow reserve may not be adequate
B) Endothelial dysfunction:
Impaired release of endothelial vasodilators by atherosclerosis (e.g. NO and Prostacyclins) leads to:
i. Inappropriate coronary vasoconstriction:
- During exercise and stress: Constrictor effect of catecholamines released cannot be outweighed
- occurs in the setting of prinzmental’s/variant/vasospastic angina
- During plaque rupture and thrombus formation: Constrictor effect of platelet products (serotonin, ADP) cannot be outweighed
- occurs in the setting of unstable angina
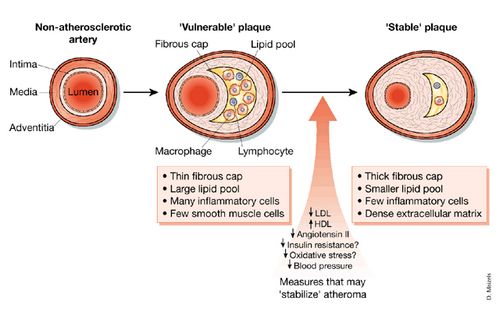
Factors predisposing to rupture of atherosclerotic plaques:
- Vulnerable or high risk plaque (Instrinsic instability): Large lipid core, thin fibrous caps, high density of macrophages and T lymphocytes, relative paucity of smooth muscle cells and increase in plaque neovascularity and intraplaque hemmorhage
- Physical stresses on atherosclerotic lesion: blood pressure, heart rate and force of ventricular contraction (exercise or emotional stress)
ii. Loss of normal antithrombotic properties
C) Non-atherosclerotic causes of ischemia:
i. Decreased coronary perfusion pressure: Hypotension (shock)
ii. Decreased blood oxygen content: Severe anemia, Pulmonary diseases
iii. Significant increase in myocardial oxygen demand: tachycardias, acute hypertension, severe aortic stenosis
iv. Coronary abnormalities:
- Emboli: Infective endocarditis, Artificial heart valves
- Inflammation: Vasculitic syndromes
- Severe transient coronary spasm: Primary, Cocaine-induced
- Congenital abnormalities, trauma, radiation or aneurysm
Risk Factors of Ischemic Heart Disease
A. Modifiable
- Smoking
- Cholesterol:
- elevated LDL
- low HDL (<40 mg/dl)
- Arterial hypertension
- Diabetes mellitus
- Physical inactivity
- Dietary factors:
- PUFA deficient diets
- Low Vitamin C and E
- Obesity (BMI ≥30 kg/m2)
- Stress factors
- Fibrinogen and factor VII
B. Non-modifiable
- Family history of premature CAD
- <55 yrs for male
- <65 yrs for female
- Age and gender:
- Male ≥ 45 years
- Female ≥ 55 years
- Genetic factors: operate in hyperlipidemia, plasma fibrinogen concentration and other coagulation factors, some of which are modifiable by lifestyle changes
C. Non-conventional
- Apolipoprotein B and Altered lipoprotein (a)
- C-reactive protein (CRP)
- Hyperhomocysteinemia
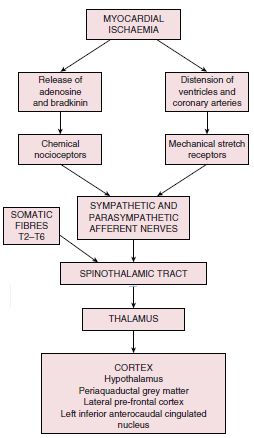
Pain Mechanism in Ischemic Heart Disease
Pathophysiology of Silent Myocardial Infarction (SMI)
1. Cardiac autonomic neuropathy (CAN):
- Damage of afferent autonomic nerves – seen in Diabetes
- Associated with postural hypotension
2. Gating mechanisms:
- Afferent signals converge onto gates, situated at the dorsal horn of the spinal cord and in the thalamus, and compete with other incoming impulses for interpretation by pain centres in the thalamus.
- Higher brain centres are responsible for prioritisation of these messages by delivering an equal descending inhibitory stimulus that suppresses incoming signals that are not perceived as acutely beneficial.
- An imbalance in this equilibrium is set to reveal the perception of pain.
- This theory suggests that pain centres may focus on another aspect of sensory mechanisms during SMI such as dyspnoea.
3. Individual pain threshold:
- Increased pain threshold in some individuals
- Higher post-exercise beta-endorphins level in some individuals
4. Personality type:
- Typical type A personality are likely to have silent MI, while depressed patients are likely to have angina
Mechanism of chest pain/discomfort: Metabolic products such as lactate, serotonin and adenosine accumulate locally and may activate peripheral pain receptors in the C7 to T4 distribution.
Spectrum of Myocardial Dysfunction Following Ischemia
1. Rapid and full recovery: after a brief episode of angina
2. Prolonged contractile dysfunction without myocyte necrosis with potential recovery of normal function:
- Stunned myocardium: Delayed recovery of systolic function despite recovery of adequate perfusion
- Due to myocyte calcium overload and accumulation of oxygen-derived free radicals during ischemia
- Hibernating myocardium: Chronic ventricular contractile dysfunction in response to a persistently reduced blood supply (multivessel CAD)
- Reversible if appropriate blood flow is restored
3. Myocardial infarction: Irreversible myocyte necrosis
| Feature | Time |
| Onset of ATP depletion | Seconds |
| Loss of contractility | < 2 min |
ATP Depletion
| 10 min 40 min |
| Irreversible cell injury | 20-40 min |
| Microvascular injury | >1 hour |
Pathophysiology of Infarction
A) Early changes in MI (minutes to days):
- Hypoxia
- Shift to anaerobic metabolism
- Impaired glycolysis and ATP production → Impaired contractile protein function
- Systolic dysfunction – loss of synchronous myocyte contraction → Compromised cardiac output
- Diastolic dysfunction – reduced ventricular compliance & elevation of ventricular filling pressures
- Lactate accumulation and pH reduction
- Na-K-ATPase impairment (impaired ATP production):
- ↑ Intracellular Na → Intracellular edema
- ↑ Extracellular K → Alteration in transmembrane potential → Electrical instability and susceptibility to arrhythmias
- ↑ Intracellular Ca → Activation of degradative lipases and proteases → Tissue necrosis
- Acute inflammatory response with neutrophil infiltration and further tissue damage
B) Late changes in MI (days to weeks):
- Resorption of irreversibly injured/dead myocytes by macrophages:
- Structural weakness of ventricular wall → Susceptibility to myocardial rupture
- Fibrosis and scarring
- Ventricular remodeling:
- Infarct expansion – thinning and dilation of necrotic tissue without additional necrosis
- Increased ventricular wall stress
- Further impairment in systolic contractile function
- Increased likelihood of aneurysm formation
- Remodeling of non-infarcted ventricle:
- Dilation due to overwork in response to increased wall stress (when reaches beyond limits of Frank-Starling’s law → heart failure and predisposition to arrhythmias)
- Infarct expansion – thinning and dilation of necrotic tissue without additional necrosis
Pathophysiology of Complications of Myocardial Infarction
1. Impaired contractility:
- Stasis → Ventricular thrombus → Systemic emboli
- Hypotension → Coronary hypoperfusion → ↑Ischemia → Cardiogenic shock
- Congestive heart failure
2. Tissue necrosis:
- Involvement of conduction pathway → Conduction defects and bradycardias
- Right coronary artery occlusion → Infarction of AV node and conduction system above bundle of His OR increased vagal tone → Sinus bradycardia, first degree heart block and type 1 second degree heart block
- Anterior infarcts → Infarction and necrosis of bundle branches in septum → Complete heart block\
- Defects in conduction system below bundle of His → type 2 second degree heart block
- Transmural necrosis of interventricular septum → VSD
- Papillary muscle involvment → Mitral or Tricuspid regurgitation → Congestive heart failure
- Necrosis of non-septal ventricular wall:
- No scar → Ventricular aneurysm → Stasis
- Scar → Ventricular wall rupture → Cardiac tamponade
3. Irritability of viable tissue adjacent to infarct:
- Arrhythmias
4. Pericardial irritation:
- Pericarditis
- Post-infarction fibrinous pericarditis (Dressler’s syndrome)
5. Congestive heart failure → Distension of atria → Atrial fibrillation