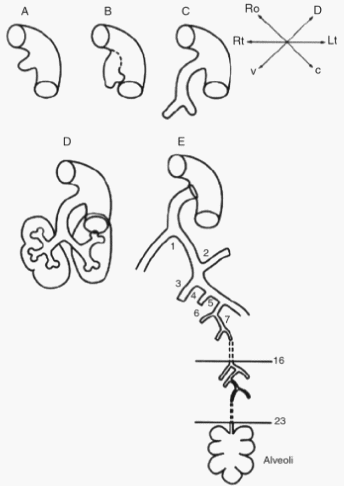
Remember the mnemonic – “Every Premature Child Takes Air“. The development of lungs comprises of 5 distinct stages:
- Embryonic (3-8 weeks, i.e. embryonic period)
- Pseudoglandular (5-16 weeks)
- Canalicular (16-26 weeks)
- Terminal saccular (26-36 weeks)
- Alveolar (36 weeks to 40 weeks and continues to childhood)
The first and last stages, i.e. Embryonic and Alveolar stages are almost 5 weeks in duration (but alveolar stage continues after birth to childhood) and the middle 3 stages, i.e. Pseudoglandular, Canalicular and Terminal saccular stages are almost about 10 weeks in duration. This is an easy way to remember the stages of lung development in fetus.
| Stage | Developmental weeks | Airway | Lining cell |
| Embryonic | 3-8 | Formation of respiratory diverticulum (from foregut endoderm in 4th week) to fromation of major bronchopulmonary segments Depends on factors from the surrounding mesoderm:
| |
| Pseudoglandular | 5-16 | Formation of all of the conducting airways:
16 airway generations in humans are completed by 16 weeks. No respiratory bronchioles or alveoli – Respiration not possible. | Simple columnar epithelium – resembles exocrine gland. |
| Canalicular | 16-26 | Respiratory bronchioles and alveolar ducts. Surrounding mesoderm have prominent capillary network. Few terminal sacs towards the end of the stage – may rarely survive with intensive care | Simple cuboidal epithelium. |
| Terminal saccular | 26-37 (birth) | Terminal sacs or Primitive saccules or Primitive alveoli Separated from eachother by primary septa. Surrounding mesoderm have rapidly proliferating capillary network – make close contact with walls terminal sacs (blood-air barrier) | Type I pneumocytes: gas exchange Type II pneumocytes: pulmonary surfactant formation (contain lamellar bodies that store surfactant)
|
| Alveolar | Birth to childhood (8 years) | Terminal sacs partitioned by secondary sepatae – adult alveoli. Exponential rise in alveoli due to secondary septation contributes to increase in lung volume after 36 weeks. | Type I and Type II pneumocytes |
Derivatives of Endoderm of respiratory diverticulum:
- Epithlial lining of tracheobronchial tree and alveoli
- Glands of larynx, trachea and bronchi
Derivatives of surrounding splanchnic mesoderm derivatives:
- Connective tissue
- Cartilages
- Smooth muscles
- Capillaries
Clinical Correlate
Aeration at birth
At birth, the lungs are approximately half-filled with the fluid secreted by fetal lung epithelium via Cl2 transport using cystic fibrosis
transmembrane protein (CFTR). The fluid is cleared by:
- Through the mouth and nose by pressure on thorax during vaginal delivery.
- Into pulmonary vessels.
- Into lymphatic vessels.
Lungs of the stillborn babies will sink in water because they are not areated and contain water rather than air. This is an important application in forensic medicine.
Pulmonary agenesis
- Complete absence of a lung or a lobe and its bronchi due to failure of bronchial buds to develop.
- Unilateral agenesis is compatible with life.
Pulmonary aplasia
- Absence of lung tissue but presence of a rudimentary bronchus.
Pulmonary hypoplasia
- Underdevelopment characterized by markedly reduced lung volume.
- Common associations:
- Right sided obstructive congenital heart defects
- Congenital Diaphragmatic Hernia (CDH)
- Bilateral renal agenesis causing oligohydramnios – may be due to reduced hydraulic pressure in the lungs and its consequential effects on lung calcium regulation.
Hyaline Membrane Disease (HMD)
Results from inadequate surfactant function:
- deficiency in Surfactant Protein B
- inadequate production of surfactant by type II pneumocytes (premature newborns)
- inadequate development of type II pneumocytes (prolonged intrauterine asphyxia/hypoxia – maternal smoking, compromised cardiovascular function in mother, uterine artery obstruction)
As a result, the airways collapse and become inflamed, resulting in the deposition of a glassy, proteinaceous film, or “hyaline membrane” on the alveolar surface that impedes gas exchange.
Antenatal corticosteroids accelerate morphologic development of type I and type II pneumocytes – when lungs have reached a developmental stage that is biologically responsible to corticosteroids.
Benefits were found when treatment was started between 26 and 35 weeks of gestation. No benefits were demonstrated for treatment commenced on infants born, before 26 weeks of gestation.