DEFINITION OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
According to GOLD, COPD is a common preventable and treatable (not curable) disease characterized by:
- Persistent airflow limitation (Post-bronchodilator FEV1/FVC < 0.7), usually progressive (excludes Asthma)
- Due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.
Chronic bronchitis, defined as the presence of cough and sputum production for atleast 3 months in each of 2 consecutive years, is not necessarily associated with airflow limitation.
Emphysema is defined as a distension of airspaces distal to the terminal bronchiole (i.e. acinus) with destruction of the alveolar septa but without obvious fibrosis.
Acute Exacerbation of COPD (AECOPD) is defined as an acute event characterized by worsening of patient’s respiratory symptoms (dyspnea, cough, sputum production) that is beyond normal day-to-day variation and leads to change in medication. They present with increased respiratory rate, increased wheezes and diffuse non-localized crackles.
- Etiology of AECOPD is multifactorial – both infectious and non-infectious; most common infectious cause is viral (influenza, parvovirus, etc.)
Other obstructive airway diseases are:
- Reversible: Bronchial Asthma
- Poorly reversible: Bronchiectasis, Cystic fibrosis, Obliterative bronchiolitis
RISK FACTORS (ETIOLOGY) OF COPD
- Tobacco smoke
- Indoor air pollution
- Occupational exposures
- Outdoor air pollution
- Genetic factors:
- alpha-1 antitrypsin deficiency
- Other associations: gene encoding MMP-12 and glutathione S-transferase
- Age and sex: Aging and female sex increase COPD risk
- Affected lung growth and development during gestation and childhood: low birth weight, respiratory infections, etc.
- Low socio-economic status
- Asthma and airway hyper-reactivity
- Infections
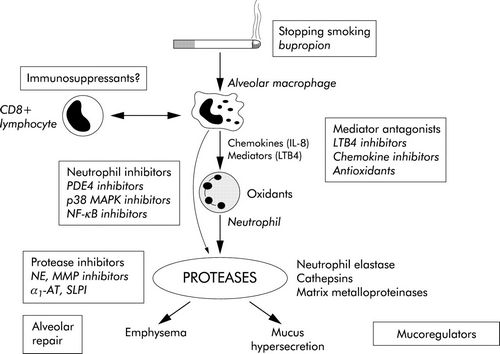
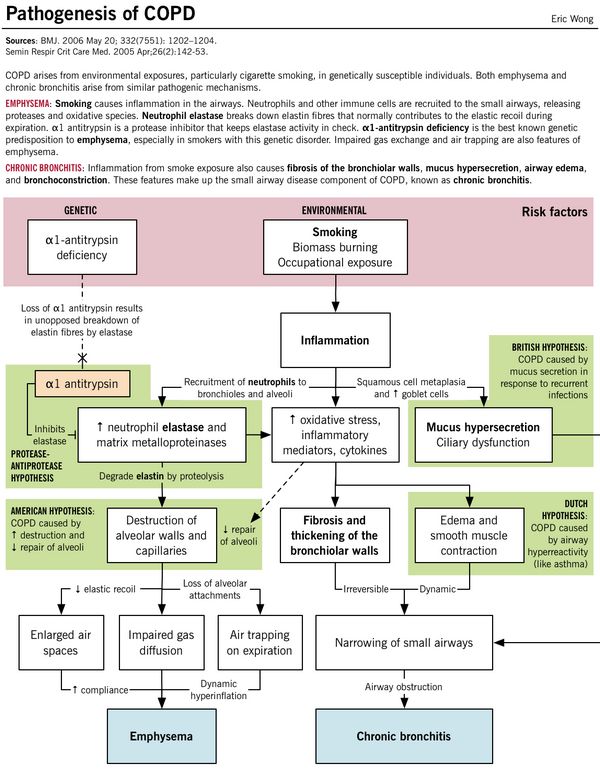
Cigarette Smoking: Smoking is associated with a variety of abnormalities of respiratory system, predisposing to COPD –
- Sluggish ciliary movement
- Bronchoconstriction
- Hypertrophy and hyperplasia of mucus-secreting glands (Ratio of thickness of submucosal glands to that of bronchial wall is expressed as Reid index; normal 0.44 +/- 0.09; >0.51 in chronic bronchitis)
- Release of inflammatory mediators in lungs (Inflammatory response is initiated by NF-Κß and Activator Protein-1 (AP-1)
- Produces high concentration of ROS (superoxide, hydrogen peroxide, hypochlorous acid) which is responsible for tissue damage and activation neutrophils and eosinophils
- Release of proteolytic enzymes from PMN leucocytes
- Inhibition of function of alveolar macrophages
Passive smoking and Maternal smoking plays important role in the development of COPD.
α1 antitrypsin deficiency:
- Alpha-1 antitrypsin is a serine protease inhibitor (SERPIN) secreted by the liver into the blood which inhibits the enzyme neutrophil elastase from damaging the lung tissue.
- Protease-antiprotease hypothesis: Deficiency of this alpha-1 antitrypsin leads to unopposed elasteolysis (destruction of the elastin fibers in alveolar walls) and development of early emphysema.
Unilateral emphysema or McLeod’s syndrome occurs as a complication of severe childhood infections caused by rubella or adenovirus, and congenital lobar emphysema is a developmental abnormality affecting newborn children.
Mnemonic:
a. American hypothesis: Airway remodelling (alveolar destruction increased and repair decreased)
b. British hypothesis: Booger (mucus) hypersection in the respiratory tract caused by infections
c. Dutch hypothesis: Duplicate of asthma (airway hyper-responsiveness)
PATHOPHYSIOLOGY OF COPD
PATHOLOGIC TYPES OF EMPHYSEMA
1. Centriacinar (Centrilobular): associated with smoking – limited to respiratory bronchioles (spares alveoli); more prominent in upper lung zones
2. Panacinar: associated with alpha-1 antitrypsin deficiency; both central and peripheral portion of acinus involved (including alveoli); more prominent in lower lung zones
3. Paraseptal: involves only distal acinus; found near the pleura and may cause spontaneous pneumothorax
COMPARISON OF PATHOLOGY OF EMPHYSEMA AND BRONCHITIS
| Emphysema | Chronic bronchitis | |
| Pathogenesis |
|
|
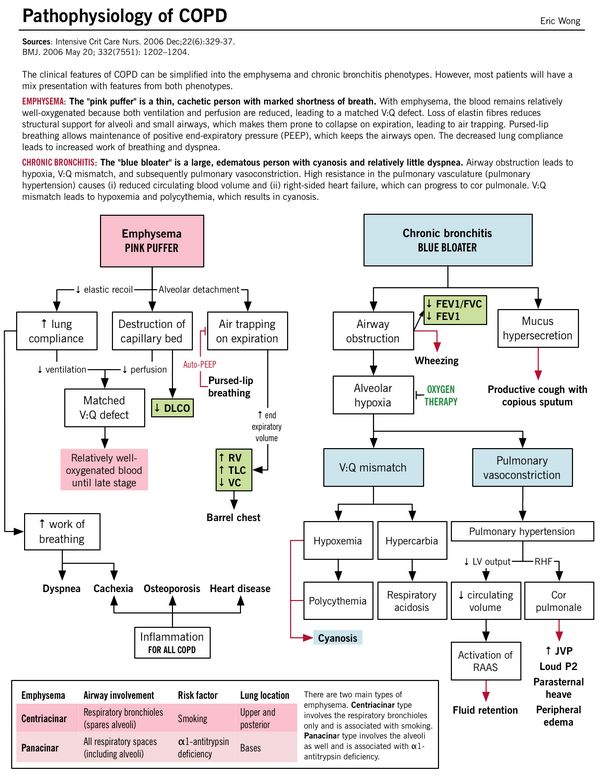
| Pathophysiology | Parenchymal destruction and Matched V/Q defect: Alveolar septa destruction along with capillary walls resulting in Matched V/Q defect (areas of low ventilation are also the areas of low perfusion).
Despite Matched V/Q defect mild hypoxia develops: As hyperventilation develops and cardiac output drops – areas of poor blood flow in relatively well oxygenated areas. Cachexia: At the pulmonary level, the low cardiac output leads to pulmonary cachexia; which induces weight loss and muscle wasting. This gives these patients the characteristic “pink-puffer” appearance. |
Small airway inflammation and obstruction: Parenchyma are relatively less damaged.
V/Q mismatch: Obstruction without capillary wall destruction leads to increased perfusion in poorly ventilated areas leading to significant hypoxia with compensator increase in cardiac output and polycythemia. Severe hypoxia and hypercarbia: Chronic V/Q mismatch leads to decreased oxygenation/deoxygenation of the blood resulting in hypoxemia and increased CO2 retention (respiratory acidosis) – leads to pulmonary artery vasoconstriction resulting in increased right ventricular pressure (pulmonary hypertension) and failure (cor pulmonale) |
COMPARISON OF CLINICAL FEATURES AND INVESTIGATIONS OF EMPHYSEMA AND CHRONIC BRONCHITIS
| Emphysema (Pink Puffer – type A) | Chronic Bronchitis (Blue bloater – type B) | |
| Dyspnea | Early onset progressive dyspnea (“puffing”): Air trapping makes each breath less efficient which is compensated by:
This inturn, leads to respiratory muscle fatigue and flattening of the diaphragm impairing it’s function – which adds to dyspnea |
Late in onset – it is due to airflow obstruction |
| Cough | Mild cough (after dyspnea starts) – due to irritation of smaller airway | Before dyspnea starts – “morning cough” that progresses in frequency, severity and duration (all round the year) |
| Sputum | Scanty and mucoid | Copious sputum (produced by goblet cells) |
| Cyanosis | No (Pink) – Matched V/Q defect; no hypoxemia | Cyanotic (Blue) – Mismatched V/Q defect; hypoxemia |
| Appearance | Cachectic – Wasting due to loss of skeletal muscle and subcutaneous fat (inflammatory cytokines and anorexia) | Bloaters – Edematous due to right ventricular failure (cor pulmonale) |
| Inspection and Palpation | Respiratory distress – tripod position, tachypnea, use of accessory muscles
Prevent collapse by maintaining high intra-bronchial pressure: Prolonged expiration through “pursed lips”, Expiratory grunt Due to chronic use of accessory muscles (sternocleidomastoid and scalene which are hypertrophied and pulls thorax in AP and upward direction): · Barrel-shaped chest (Normal AP to transverse diameter of chest is 5:7; increased AP diameter in barrel-shaped chest) · Prominent angle of louis · Horizontally and widely placed ribs · Widened subcostal angle (normal is 70⁰) Hoover’s sign: Flattened diaphragm which contracts inwards instead of downwards, thereby paradoxically pulling the inferior ribs inwards with its movement (instead of outwards during normal respiration) Campbell’s sign: exaggerated tracheal descent during inspiration (increased work of breathing where movements of chest wall, diaphragm and muscles is transmitted to trachea) Dahl’s sign: Symmetric, slanting regions of hyperpigmentation the thighs – from patients with COPD resting in the tripod position (elbows on thighs) Apical impulse – invisible or feeble (may be felt at subxiphoid region because heart is vertical and rotated Tactile fremitus – Diminished |
At rest, no respiratory distress – no tachypnea, no use of respiratory muscle
Usually, no deviation from expected findings Tactile fremitus – Normal |
| Percussion | Hyper-resonant over lungs
Cardiac dullness reduced or obliterated Liver dullness pushed down or absent |
Normally resonant over lungs
Liver dullness and cardiac dullness in normal position |
| Auscultation | Diminished intensity of breath sound bilaterally
Vesicular breath sound with prolonged expiration (>6 seconds) Scattered, faint, high-pitched, end-expiratory rhonchi Dimished audibility of heart sounds |
Vesicular breath sound with prolonged expiration
Wheezes – airway obstruction Crepitation – gurgling sound due to mucus hypersecretion in airways (disappear or change in intensity or location after coughing) |
| Complications | ||
| Pulmonary hypertension (RVH) | Late and mild | Early and severe (Visible and palpable pulmonary artery pulsations, sustained left parasternal heave, Epigastric pulsations, palpable and loud P2) |
| Right ventricular failure (Cor pulmonale) | Late and often terminal | Repeated episodes (Peripheral edema, raised JVP, tender hepatomegaly, S3 of right ventricular origin), Functional Tricuspid regurgitation (TR – distended neck veins, pansystolic murmur accentuated during inspiration) |
| Respiratory failure | Late and often terminal | Repeated episodes (Type I or Type II); CO2 narcosis manifest as clouding of consciousness, altered behavious, drowsiness, headache, papilledema, bounding pulse and asterexis (flapping tremor) |
| Mucopurulent relapses (S.pneumoniae, H.influenzae, M.catarrhalis) | Less frequent | More frequent (fever and frankly purulent copious sputum) |
| Specific | Pulmonary bullae (from ruptured alveolar walls) – usually located subpleurally along anterior border of lungs which can rupture causing spontaneous pneumothorax | Secondary polycythemia – stimulated by hypoxemia |
| Non-specific | Anemia, Osteoporosis, Depression, Increased Cardiovascular risk | |
| Investigations | ||
| Hematocrit | Normal | Increased (Polycythemia) |
| PaO2 (ABG) | Normal to low | Low (Hypoxia) |
| PaCO2 (ABG) | Normal | High (Hypercarbia) |
| PFT | ↓FEV1 (<12% post-bronchodilator reversibility), ↓FVC, ↓FEV1/FVC, ↓PEF, ↑TLC, ↑FRC, ↑RV | |
| Diffusing lung capacity (DLCO) – PFT | Reduced | Normal |
| Chest X-ray | Hyperinflation (low set diaphragm, translucency increased, loss of peripheral vascular markings, widely placed and horizontal ribs) , Bullae and tubular heart, prominent pulmonary artery shadow at hilum | Increased broncho-vascular markings and cardiomegaly |
| ECG | ECG changes in COPD | |
Key indicators for considering a diagnosis of COPD
Consider COPD, and perform spirometry, if any of these indicators are present in an individual over 40.
- Dyspnea (progressive, worse with exercise and persistent)
- Chronic cough (may be intermittent and unproductive, recurrent wheeze)
- Chronic sputum production
- Recurrent lower respiratory tract infections
- History of risk factors (genetic, developmental abnromalities, tobacco smoke, indoor smoke, occupational exposure)
- Family history of COPD and/or childhood factors (low birth weight, childhood respiratory infections)
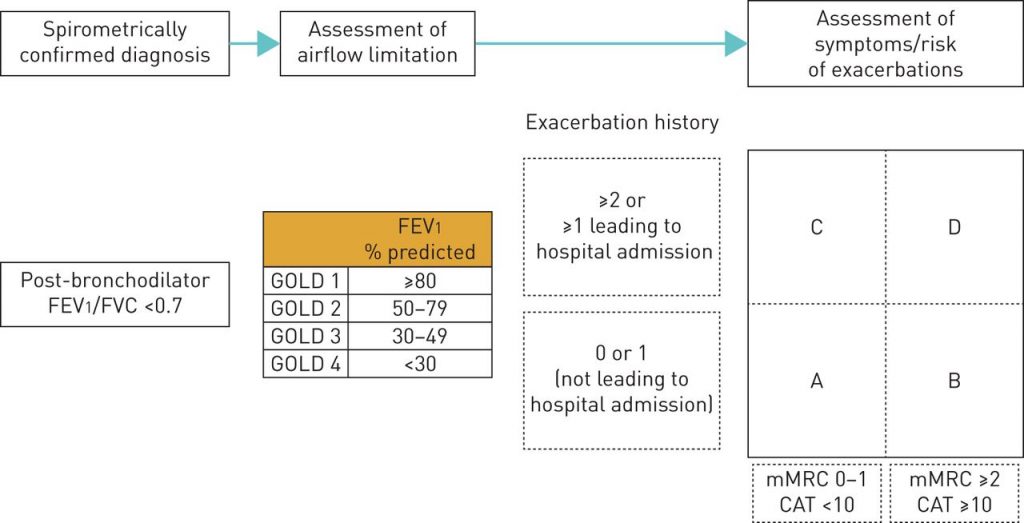
CLASSIFICATION OF SEVERITY OF AIRFLOW LIMITATION IN COPD
Based on post-bronchodilator FEV1 in patients with FEV1/FVC < 0.7
- GOLD 1 (Mild): FEV1 ≥ 80% predicted
- GOLD 2 (Moderate): 50% ≤ FEV1 < 80% predicted
- GOLD 3 (Severe): 30% ≤ FEV1 < 50% predicted
- GOLD 4 (Very severe): FEV1 <30% predicted
It should be noted that there is only a weak correlation between FEV1, symptoms and impairment of a patient’s health status.
COMBINED ASSESSMENT OF COPD
CAT (COPD Assessment Test) Questionnaire:
Assessment of severity of symptoms with 8 questions – each scored in the range of 0 to 5 (O being no symptoms to 5 being maximum symptoms)
Mnemonic: COPD
- Cough
- Chest constriction (tightness)
- Confidence in leaving home
- Occupation (Activity) limitation at home
- Phlegm (mucus)
- Psyche (Energy)
- Disturbed sleep
- Dyspnea on walking uphill or 1 flight of stairs
mMRC (modified Medical Research Council) Dyspnea scale:
0 – Breathless only on exertion
1 – Breathless on hurrying on level ground or walking slight uphill
2 – Walk slower on level ground (compared to those of same age) because of breathlessness OR Have to stop on walking on own pace on level ground
3 – Breathless after walking ~ 100 m or after few minutes on level ground
4 – Too breathless to leave house or Breathless on dressing/undressing
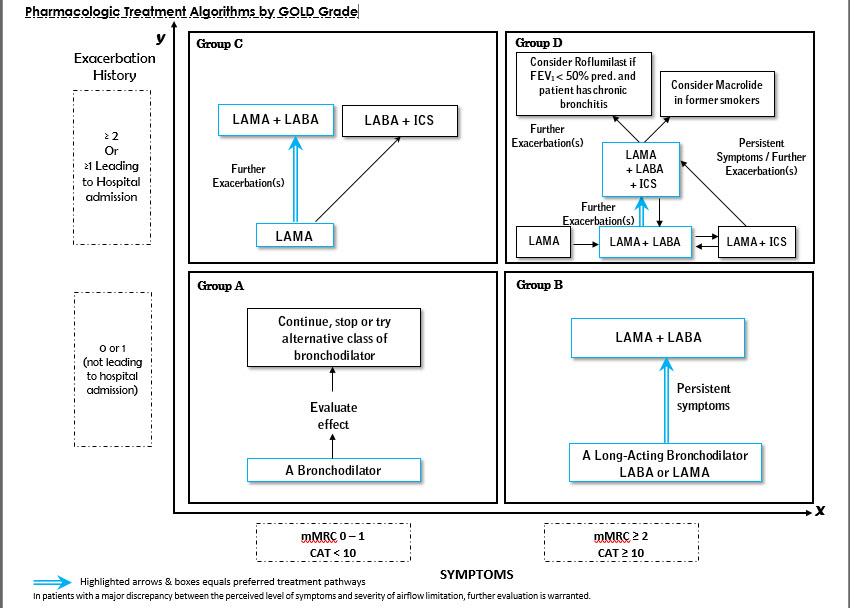
Refined ABCD Assessment of COPD
First step: Spirometry to grade airflow limitation
Second step: Assessment of dyspnea using mMRC or symptoms using CAT
Final step: History of exacerbations (including prior hospitalizations) should be recorded.
MANAGEMENT OF COPD
1. Treatment of Acute Exacerbation of COPD (AECOPD) if present
Classification of AECOPD:
a. No respiratory failure: Respiratory rate: 20-30 breaths per minute; no use of accessory respiratory muscles; no changes in mental status; hypoxemia improved with supplemental oxygen given via Venturi mask 28-35% inspired oxygen (FiO2); no increase in PaCO2.
b. Acute respiratory failure – non-life-threatening: Respiratory rate: > 30 breaths per minute; using accessory respiratory muscles; no change in mental status; hypoxemia improved with supplemental oxygen via Venturi mask 25-30% FiO2; hypercarbia i.e., PaCO2 increased compared with baseline or elevated 50-60 mmHg.
c. Acute respiratory failure – life-threatening: Respiratory rate: > 30 breaths per minute; using accessory respiratory muscles; acute changes in mental status; hypoxemia not improved with supplemental oxygen via Venturi mask or requiring FiO2 > 40%; hypercarbia i.e., PaCO2 increased compared with baseline or elevated > 60 mmHg or the presence of acidosis (pH < 7.25).
Investigations:
- PEF measurement
- Oxygen saturation with or without ABG (in moderate to severe cases)
Sputum cultures are not routinely recommended as these patients are often colonized with respiratory pathogens. It may be helpful in end-stage COPD, frequent exacerbations or bronchiectasis to determine colonizations with gram negative organisms like Pseudomonas aeruginosa.
Indications for ICU admission:
- Severe dyspnea that responds inadequately to initial emergency therapy.
- Changes in mental status (confusion, lethargy, coma)
- Persistent or worsening hypoxemia (PaO2 <40 mmHg) and/or severe/worsening respiratory acidosis (pH <7.25) despite supplemental oxygen and non-invasive ventilation.
- Need for invasive mechanical ventilation.
- Hemodynamic instability – need for vasopressors.
a. Oxygen:
- Aim: Oxygen saturation of 88-92%
- Be careful – high flow rates can worsen hypercapnia
- Increased dead-space due to aggravation of V/Q mismatch due to release of hypoxic pulmonary vasoconstriction
- Loss of hypoxic respiratory drive
- Co2 binding capacity decreases as hemoglobin oxygen saturation increases (Haldane effect)
b. Bronchodilators:
- Nebulized Short Acting Beta-Adrenergics/SABAs (Salbutamol 2.5 mg i.e. 0.5 ml) and Anticholinergics (Ipratropium 0.5 mg i.e. 2.5 ml) every 20 minutes for initial 1-2 hours
- SABAs: fast onset of action but short-lived
- Anticholinergics: delayed onset of action but prolonged effect
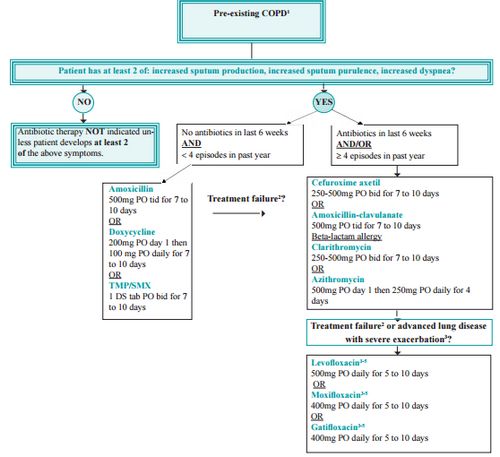
c. Antibiotics: Indicated if 2 out of 3 symptoms of exacerbation
- Commonest organisms are: Viral (Influenza, Parainfluenza), Bacterial (S.pneumoniae, H.influenzae, M.catarrhalis), Atypical organisms (Mycoplasma and Chlamydia pneumoniae), Recent hospitalizations and frequent exacerbations i.e. 4 or more in last 1 year and severe exacerbation (P.aeruginosa)
- Agents not recommended in AECOPD are:
- Cephalexin (poor activity against penicillin intermediate/resistant S.pneumoniae, no activity against hemophilus and moraxella)
- Cefaclor (no activity against penicillin intermediate/resistant S.pneumoniae, marginal activity against hemophilus)
- Cefixime (no activity against penicillin intermediate/resistant S.pneumoniae, excellent activity against hemophilus)
- Ceftriaxone (not recommended for routine use due to potential for increased resistance to 3rd generation cephalosporins)
- Erythromycin (poor activity against hemophilus and moraxella)
- Clindamycin (no activity against hemophilus and moraxella)
- No antibiotics in <6 wks and no frequent exacerbation (<4/year):
- Amoxicillin 500 mg PO TID for 7-10 days OR
- Doxycycline 200 mg PO stat then 100 PO OD daily for 7-10 days OR
- TMP/SMX 1 DS tablet PO BD for 7-10 days
- Antibiotics in <6 wks and/or frequent exacerbations:
- Cefuroxime 250-500 mg PO BD for 7-10 days OR
- Amoxicillin-Clavulanate 500 mg PO TID for 7-10 days OR
- Clarithromycin 250-500 mg PO BD for 7-10 days OR
- Azithromycin 500 mg PO stat then 250 mg PO OD for 4 days
- Treatment failure or advanced lung disease with severe exacerbation:
- Levofloxacin 500 mg PO OD for 5-10 days OR
- Moxifloxacin 400 mg PO OD for 5-10 days OR
- Gatifloxacin 400 mg PO OD for 5-10 days
Treatment failure – Clinical deterioration after 72 hours of antibiotic therapy or no improvement after 7-10 days of antibiotic therapy
For severe lung disease – Levofloxacin, Moxifloxacin or Gatifloxacin for 10 days (reserved also for treatment failure to minimize development of antibiotic resistance)
Ciprofloxacin – suboptimal coverage for S.pneumoniae; for documented P.aeruginosa infection Ciprofloxacin 750 mg BD for 10 days can be used
d. Corticosteroids:
For patients with advanced lung disease of less severe lung disease with severe exacerbation:
- Oral prednisolone: start at 0.5-1 mg/kg/day and taper slowly OR
- IV methylprednisolone: 40-125 mg every 8-12 hours OR
- IV hydrocortisone: 100 mg every 6-8 hours
They shorten the recovery time and improve lung function (FEV1) and hypoxemia.
Limit the duration of therapy to: 5-7 days
e. Methylxanthines: are not recommended due to side effect profiles
f. Diuretics: in patients with gross right ventricular failure (cor-pulmonale)
g. Non-invasive ventilation (NIV): Indications – atleast one of the following –
- Respiratory acidosis
- Severe dyspnea with clinical signs suggestive of respiratory muscle fatiue, increased work of breathing or both such as use of accessory muscles of respiration, paradoxical motion of abdomen, or retraction of the intercostal spaces.
- Persistent hypoxemia despite supplemental oxygen therapy.
h. Invasive mechanical ventilation: Indications –
- Unable to tolerate NIV or NIV failure
- Status post-respiratory or cardiac arrest
- Diminished consciousness, psychomotor agitation inadequately controlled by sedation
- Massive aspiration or persistent vomiting
- Persistent inability to remove respiratory secretions
- Severe hemodynamic instability without response to fluids and vasoactive drugs
- Severe ventricular or supraventricular arrhythmias
- Life-threatening hypoxemia in patients unable to tolerate NIV
2. Management of Stable COPD
In some patients, initial therapy with LABA/ICS may be the first choice; this treatment has the greatest likelihood of reducing exacerbations in patients with blood eosinophil counts >/= 300 cells/microlitres. LABA/ICS may also be the 1st choice in COPD patients with a history of asthma.
a. Bronchodilators:
- Short-acting (4-6 hours for ß2 adrenergics and 6-8 hours for anticholinergics ): Inhaled ß2 adrenergics (salbutamol 100-200 mcg e.g. ventolin, terbutaline 400-500 mcg), Inhaled anticholinergics (ipratropium 20-40 mcg), Oral ß2 adrenergics (salbutamol 2 or 4 mg e.g. ventolin, terbutaline 2.5 or 5 mg); Combination of salbutamol/ipratropium (100/20 mcg)
- Long-acting (12 hours for ß2 adrenergics and 24 hours for anticholinergics: Inhaled ß2 adrenergics (salmeterol 25-50 mcg, formoterol 4.5-12 mcg), Inhaled anticholinergics (tiotropium 18 mcg e.g. tiova)
Other medications:
- Short acting beta adrenergics: Fenoterol, Levalbuterol
- Short acting anticholinergics: Oxitropium
- Long acting beta adrenergics (once daily): Indacaterol, Oladaterol, Vilanterol
- Long acting anticholinergics: Aclidinium, Glycopyrronium, Umeclidinium
b. Inhaled corticosteroids:
- Beclomethasone 50-400 mcg
- Budesonide 100, 200, 400 mcg
- Fluticasone 50-500 mcg
Foracort is a combination of formoterol (fixed 6 mcg) and budesonide (100, 200 or 400 mcg)
c. Oral methylxanthines:
- Aminophylline 200-600 mg pill
- Theophylline 100-600 mg pill
- Doxofylline 400 mg (PO BD)
d. Systemic steroids: Prednisolone 5-60 mg (pill), Methylprednisolone 4, 8, 16 mg (pill)
- Long term use of systemic steroids is not recommended
e. Phosphodiesterase-4 inhibitor (new class): roflumilast 500 mcg (duration 24 hours)
More recent studies have shown that regular use of macrolide antibiotics may reduce exacerbation rate.
f. Symptomatic measures:
- Hot drinks or steam inhalation to liquefy sputum
- Mucolytics: bromhexine, N-acetylcysteine, carbocysteine, ambroxol, erdosteine
g. Non-pharmacological interventions:
- Regular physical activity
- Pulmonary rehabilitation: patient assessment, exercise training, education, behaviour change, nutritional intervention and psychosocial support
- Chest physiotherapy: for chronic bronchitis, those with co-existent bronchiectasis and some patients during an acute exacerbation
- Smoking cessation:
- 5 A’s of Intervention: Ask (about smoking status), Advise, Assess (willingness to attempt quit within 30 days), Assist (Refer pharmacotherapy – nicotine replacement therapy, buprobion, varenicline; Refer community cessation service), Arrange (assess smoking status every visit)
- 5 R’s of Motivation: Relevance (benefits of quiting), Risks, Rewards, Roadblocks, Repetition
h. Long-term domiciliary oxygen therapy:
Atleast 15 hours per day:
- PaO2 ≤ 55 mmHg or SaO2 <88% OR
- PaO2 >55 but <60 mmHg in the presence of pulmonary hypertension (PAP > 25 mmHg), right heart failure or polycythemia
Hypoxaemia is best screened for using pulse oximetry, however should be confirmed using arterial blood gas (ABG) measurement.
Pescribe supplemental oxygen and titrate to keep SaO2 ≥90%.
Recheck in 60-90 days to assess:
- If oxygen is still indicated.
- If prescribed supplemental oxygen in effective.
i. Treatment of pulmonary hypertension:
- Long term oxygen therapy (hypoxia is a potent pulmonary vasoconstrictor)
- Synthetic prostacyclins (epoprostenol, iloprost, treprostinil)
- Endothelin-1 receptor antagonists (bosentan)
- Phosphodiesterase-5 inhibitors (sildenafil, tadalafil)
j. Surgeries:
- Bullectomy: resection of large bulla (> 5 cm); giant bulla can be defined as those occupying ≥ 30% of hemithorax with definite displacement of adjacent lung tissue
- Lung volume reduction surgery (LVRS): resection of most severely affected areas of non-emphysematous, non-bullous lungs
- Lung transplantation: Recommended for –
- BODE index 7-10 OR ≥1 of the following:
- History of hospitalisation for exacerbations associated with acute hypercapnia
- Pulmonary hypertension or cor pulmonale or both, despite oxygen therapy
- FEV1 < 20% and either DLCO < 20% or homogeneous emphysema
BODE Index for COPD
Scoring: Add all the 4 parameters and calculate score out of 10:
- Body Mass Index (BMI)
- Points 0: BMI >21
- Points 1: BMI 21 or less
- Obstructive airway disease (FEV1)
- Points 0: FEV1 >64%
- Points 1: FEV1 50-64%
- Points 2: FEV1 36-49%
- Points 3: FEV1 <36%
- Dyspnea Index (mMRC)
- Points 0: 0-1
- Points 1: 2
- Points 2: 3
- Points 3: 4
- Exercise (Six-Minute Walk Test)
- Points 0: Walks >349 meters
- Points 1: Walks 250-349 meters
- Points 2: Walks 150-249 meters
- Points 3: Walks <150 meters
Interpretation: 4 year survival –
- 0-2: 80%
- 3-4: 67%
- 5-6: 57%
- 7-10: 18%
Updated as per GOLD 2017 Pocket Guide
Wow…too good. The best article on copd on the whole internet.good job.well done.would love to see other topics.
Very very grateful to the person who compiled all the necessary info for making this topic easily understandable.