Concentration effect
The higher the concentration of an inhaled anesthetic, the faster the alveolar concentration approaches the inhaled concentration. This is referred to as concentration effect and is clinically significant only in cases where gases are administered in high concentration:
- Nitrous oxide
- Xenon
Ostwald’s blood gas solubility coefficient: ratio of the concentration in blood to the concentration in gas that is in contact with that blood, when the partial pressure in both compartments is equal. This coefficient for nitrous oxide is 0.47. A gas with coefficient 0, i.e. totally insoluble has a very quick induction and recovery period. Similarly, nitrous oxide also has a relatively low blood gas solubility coefficeint – making the induction and recovery quick.
To understand the concept clearly, we will illustrate it with an example:
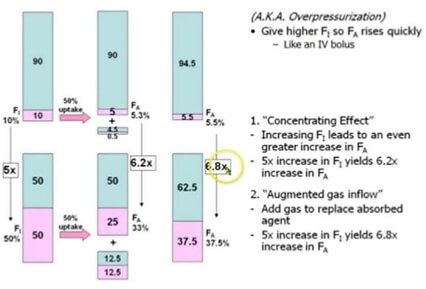
Suppose, the capacity of patients lungs is 4 l. A patient inhales 2 litres of nitrous oxide and 2 litres on oxygen, such that each have 50% concentration in the mixture. We all know that, the gas flows along the concentration gradient until they maintain equilibrium. Since, nitrous oxide diffuses very rapidly than other gases, half of the volume, i.e. 1 l of it diffuses into blood, leaving only 1 l in the alveoli. Initial 4 l (2 l of nitrous oxide and 2 l of oxygen) of alveolar volume has now been reduced into 3 l (1 l of nitrous oxide and 2 l of oxygen). So, the new concentration of nitrous oxide in alveoli is 1/3, i.e. ~ 33%. This is the first part of concentration effect and is termed as concentrating effect.
As, the alveoli is deficient of 1 l volume, this creates subatmospheric pressure in alveoli and the patient further inhales 1 l of mixture of gases containing 50% of each nitrous oxide and oxygen, i.e. 500 ml of each nitrous oxide and oxygen. Now, the volume of gases in alveoli is 4 l (2.5 l of oxygen and 1.5. l of nitrous oxide). Now, the concentration of nitrous oxide in alveoli is 1.5/4, i.e. ~ 37.5%. This is the second part of concentration effect and is termed as augmented gas inflow effect or ventilation effect.
In comparison to this, if we had used a mixture of 10% nitrous oxide and 90% oxygen – the rise in alveolar concentration of nitrous oxide with obviously be very low. It would be about 5.3% by concentrating effect and 5.5. % by augmented inflow effect which is 5 times and 6.8 times less compared to the 50:50 mixture.
So, it is clear from the above illustration – greater the inspired concentration, the more rapid the rate of rise of alveolar concentration.
Second gas effect
Second gas effect is not a different concept than the concentration effect. It is a special case of concentration effect and applies to administration of a potent anesthetic with nitrous oxide. Along with the concentration of potent agent in the alveoli via its uptake, there is further concentration via the uptake of nitrous oxide, a process called the second gas effect. Though it has low solubility in blood, about 1 litre/min of nitrous oxide enters blood in the 1st few minutes. If another potent anesthetic (second gas) is given at the same time, it also will be delivered to blood at a rate 1 litre/min higher than the minute volume and the induction will be faster.
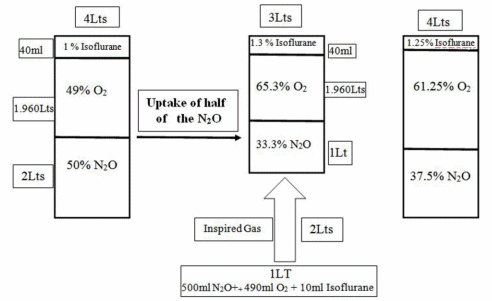
We will use the same illustration to explain the second gas effect by adding isoflurane so that the inhalation mixture is 1% isoflurane + 49% oxygen + 50% nitrous oxide. 4 l volume is inhaled by the patient, i.e. 40 ml isoflurane + 1960 ml oxygen + 2000 ml nitrous oxide.
- Concentrating effect: As, the half of nitrous oxide diffuses quickly to blood, alveolar volume reduces to 3000 ml. The new alveolar concentration of isoflurane is 40/3000 ~ 1.33%.
- Augmented inflow or ventilation effect: Due to subatmospheric pressure created in alveoli, further 1 l of mixture gas is inhaled, i.e. 10 ml isoflurane, 490 ml oxygen and 500 ml nitrous oxide. So, the new alveolar concentration gradient is (40+10)/4000 ~ 1.25%.
By second gas effect, nitrous oxide increases the alveolar concentration of a second gas like halothane, isoflurane, etc.
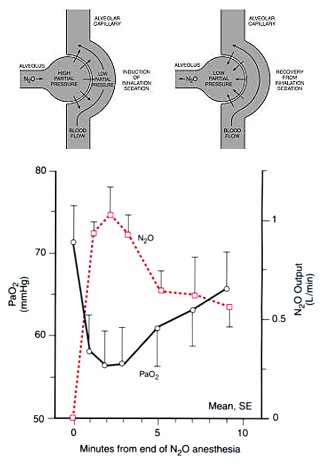
Diffusion hypoxia or Fink effect
During recovery from the anesthesia, when nitrous oxide is discontinued – large concentration of nitrous oxide diffuses back to the alveoli from the blood. This is due to low blood solubility of nitrous oxide (N2O) and results in:
- Dilution of the inspired oxygen concentration and hypoxia.
- Dilution of inspired carbon-dioxide concentration and subsequent decrease in arterial carbon dioxide concentration leading to reduction in respiratory drive.
The risk persists only for initial 3-5 minutes of discontinuing nitrous oxide. Therefore, it is appropriate to initiate recovery with 100% oxygen inhalation. It is not of much consequence if cardiopulmonary reserve is normal but may be dangerous if it is low.
Video illustrating concentration effect, second gas effect and diffusion hypoxia
These effects are very nicely explained above, but there is utter confusion in the video. In explaining concentration effect there is no need to mention Halothane. Adding N2O in a mixture of O2 and Halothane increases alveolar concentration of halothane thus increasing its concentration gradient hence its its uptake. This is 2nd gas effect. Concentration effect is not properly explained in the video.
Thank you for nice explanation.
Thank you so much , this made me understand the effects well.
A very good explanation for complex topic