Stages of Cell Cycle
Interphase
Mnemonic: Go Sally Go! Make Children
1. G1 phase
2. S phase
3. G2 phase
4. Mitosis
5. Cytokinesis
1. G1 (Gap 1) phase:
- Early and late phase divided by Restriction (R) point
- Functions:
- Preparation for DNA replication (synthesis of replication proteins cyclin D)
- Thymidine dimer repair
- Variable duration (8 hours to several days, weeks or months – most cells are in G1 phase)
- Early G1 phase: requires mitogens to proceed forward
- G0 (Quiescence): cells can exit G1 phase and enter G0 phase if mitogens are absent
- R (Restriction point): beyond the R point, cell cycle will progress even in the absence of mitogens
- Late G1 phase: mitogen is not required to proceed further
2. S (Synthesis) phase:
- DNA replication occurs
- Constant duration (6-8 hours)
- Production of Cyclin B
3. G2 (Gap 2) phase:
- Cellular growth to prepare for cell division
- 2-5 hours
Mitosis (~1 hour)
Mnemonic: PMAT
1. Prophase: Prepare for division
2. Metaphase: Meet in midline
3. Anaphase: Alone and Apart
4. Telophase: Torn and towed
Prophase: Chromatin condense into chromosomes
Metaphase: Chromosomal arrangement and attachment to spindles (alignment)
Anaphase: Pulling apart of sister chromatids (Chromosomes split)
Telophase: Chromosomal decondensation, reformation of nuclear membrane and breakdown of spindles
Cytokinesis (Cell division)
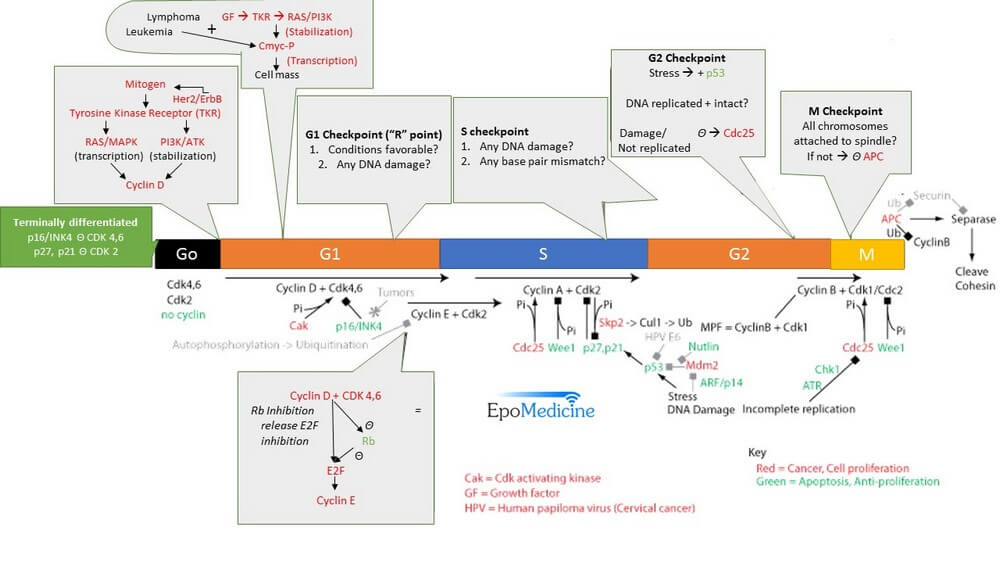
Cyclins – Cell Cycle Controllers
Cyclins (Synthesized in specific phase of cell cycle and activates CDKs) + Cyclin Dependent Kinases/CDKs (Constitutive but inactive) = Promotes cell cycle progression by phosphorylation of key proteins
- Cyclin D + CDK 4 = promotes progression of cell cycle past “R” point (G1-CDK complex)
- Cyclin E + CDK 2 = promotes progression of cell cycle past G1/S checkpoint (G1/S-CDK complex)
- Cyclin A + CDK 2 = promotes progression of cell cycle past S checkpoint (S-CDK complex)
- Cyclin B + CDK 1 = promotes progression of cell cycle past M checkpoint (M-CDK complex)
Mnemonic: To remember the match for Cyclins and CDKs – DEAB 42 21.
The phosphorylation of RB is a molecular ON-OFF switch for the cell cycle.
Cyclin D is the first cyclin to increase in the cell cycle.
The initiation of DNA replication involve the formation of an active complex between cyclin E and CDK2.
The main mediator that propels the cell beyond prophase is the cyclin B-CDK1 complex.
Proteins of the RAD and ataxia telangiectasia mutated (ATM) families act as sensors. Proteins of the CHK kinase families act as transducers.
Cell Cycle Inhibitors
CDK-cyclin activation requires phosphorylation of CDK by CDK activating complexes (CAK). 2 ways to inhibit CDK-cyclin complex are:
- Phosphorylation of CDK (at a different site than CAK) to inhibit it: Wee1
- cdc25 removes inhibitor phosphates to activate CDK-cyclin complex.
- Binding of inhibitory proteins to CDK-cyclin complex: CDK inhibitors
- Cip/Kip family components (p21,p27,p57): non-specific
- INK4a/ARF locus (inhibitor of kinase 4/alternative reading frame): p16INK4a (competitively blocks CDK4 and causes cell cycle arrest at late G1); p14ARF (prevents feedback inhibition of p53).
Cyclical degeneration of Cyclins is mediated through Ubiquitin-Proteasome pathway.
Checkpoints
Passage through a checkpoint from one cell cycle phase to the next requires a coordinated set of proteins that monitor cell growth and DNA integrity.
a. “R” (Restriction) point:
- During G1 : RB gene (Tumor suppressor) + EF2 (Transcription factor) = RB binds and blocks EF2
- Cyclin D + CDK 4,6 = Phosphorylation of RB gene = Inhibition of RB gene = Transcription of S-phase promoting genes by EF2 (e.g. Cyclin E)
b. G1/S checkpoint:
- Allows checking of DNA integrity before replication in S phase
- Cyclin E + CDK 2 allows progression through G1/S check point
- Controlled by: p53 which induces cell cycle inhibitor p21
c. G2/M checkpoint:
- Checks for damaged/incompletely replicated DNA and adequacy of cell size.
- Cells damaged by ionizing radiation activate G2/M checkpoint.
- Arrest by:
- p53-dependent mechanism: via cyclinA/CDK2 inhibition
- p53-independent mechanism: via cdc25 inactivation
d. M checkpoint:
- Checks if Chromosomes all properly attached to spindles
- Chromosomes without attachment to spindle sends signal that blocks activation of APC (Anaphase Promoting Complex) which blocks progression from metaphase to anaphase.
Cell types
Permanent
- enter G0 and cannot leave
- e.g. neurons, skeletal, cardiac muscle, RBCs
Stable (quiescent)
- enter G0 and can leave when given appropriate stimulus
- e.g. hepatocytes, lymphocytes
Labile
- never go to G0
- constant division with a condensed G1
- e.g. bone marrow, skin, gut epithelium
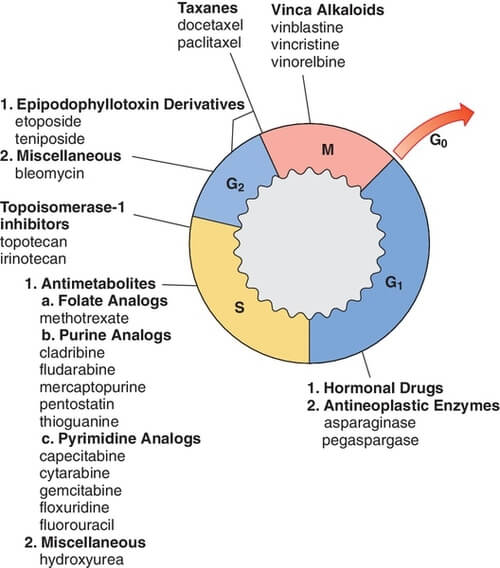
Cell cycle and Cancer Chemotherapy
Read the principles of cancer chemotherapy.
Disorders of Cell Cycle Regulatory Proteins
G1 checkpoint Inhibiting Proteins
RB – Prevents entry into S phase in the absence of Growth signals by inhibiting E2F transcription factor.
p53 – Slows cell cycle and entry into S phase in response to DNA damage by inducing p21 which inhibits CDK; if DNA repair is not possible, it upregulates BAX which disrupts BCL2 and cytochrome C is leaked out from mitochondria into cytosol leading to apoptosis.
Knudson’s 2 hit theory:
Both copies of tumor suppressor – p53 or RB gene must be knocked out for tumor formation:
- p53: Germline mutation (1st hit) + Somatic mutation (2nd hit) = Li Fraumeni Syndrome
- RB:
- Germline mutation (1st hit) + Somatic mutation (2nd hit) = Familial Retinoblastoma (Bilateral)
- Somatic mutation (1st hit) + Somatic mutation (2nd hit) = Sporadic Retinoblastoma (Unilateral)
S checkpoint Inhibiting Proteins
When DNA break is detected – tumor suppressor genes encode:
ATM (Ataxia Telangiectasia Mutated) protein: halts cell cycle and activates other proteins involved in repairing the break including BRCA 1
- Inherited mutation of ATM protein = high risk of leukemia and lymphomas
BRCA 1 (Breast Cancer 1) protein: mediate DNA repair or apoptosis
- Mutation is associated with breast and ovarian carcinoma
G2 checkpoint Inhibiting Proteins
If DNA damage is detected – p53 prevents entry into M phase
M checkpoint Inhibiting Proteins
When chromosomes are not properly attached to the mitotic spindles – MAD (Mitotic Arrest Deficient) proteins inhibit APC (Anaphase Promoting Complex and prevents entry into anaphase.

Where from you get G2 check point? I don’t know about it. If possible please send me your explanation.
The G2-phase checkpoint, also known as G2/M-phase checkpoint, has the function of preventing cells with damaged DNA, lasting from the G1 and S phases or generated in G2, from undergoing mitosis.