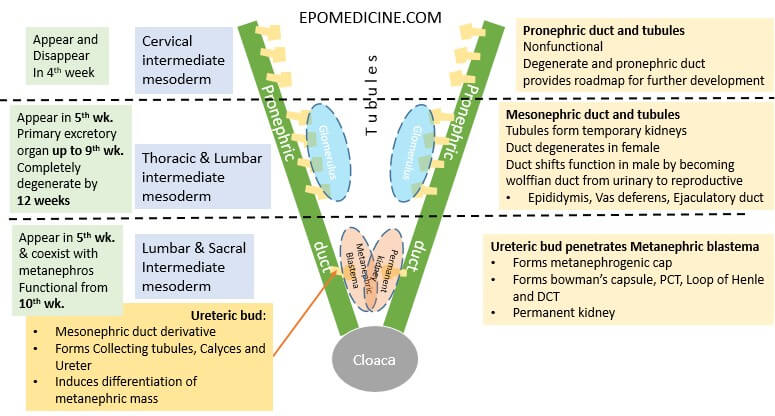
Kidney development occurs chronologically from cranial to caudal direction from urogenital ridge (intermediate mesoderm) in 3 different phases.
- Intermediate mesoderm → urogenital ridge (longitudinal elevation along dorsal body wall) → nephrogenic cord → Pronephros, Mesonephros and Metanephric mesoderm/blastema
Remember the embryology of brain – from cranial to caudal, the primordial structures are prosencephalon, mesencephalon and metalencephalon. Similar terminologies will be used here with replacement of “encephalon” with “nephros”.
Pronephros
- Cranial-most nephric structure developing from cervical intermediate mesoderm
- Pronephric ducts and tubules
- Non-functional; only lays a roadmap for further development of kidneys
- Appears and regresses during the 4th week
Mesonephros
- Middle nephric strucutre developing from thoracic and lumbar intermediate mesoderm
- Mesonephric duct and tubules
- Appears in 5th week
- Functions:
- Temporary kidney – primarily from 5 to 9 weeks
- Provides ureteric bud – part of metanephros and helps in metanephric blastema/mass/mesoderm differentiation
- Regresses completely by 12 weeks (1st trimester)
- Male – forms Wolffian duct (precursor of epididymis, vas deferens and ejaculatory duct)
- Vestigial remnants of the mesonephric duct: appendix epididymis
- Vestigial remnants of mesonephric tubules: paradidymis
- Female –
- Vestigial remnants of the mesonephric ducts: appendix vesiculosa and Gartner’s duct
- Vestigial remnants of the mesonephric tubules: epoophoron and paroophoron
- Male – forms Wolffian duct (precursor of epididymis, vas deferens and ejaculatory duct)
Metanephros
- Caudal-most nephric structure developing from lumbar and sacral intermediate mesoderm
- Ureteric bud invading into Metanephric blastema/mass/mesoderm and inducing its differentiation
- Appears in 5th week and coexists with mesonephros until it degenerates
- Functional from 10th-12th week – Distal tubules (metanephros) have conencted with Collecting ducts (ureteric bud)
- Nephrogenesis continues through 32 – 36 weeks of gestation
- Functional maturation of nephrons continue through out infancy.
WT1 expressed by the mesenchyme, makes metanephric blastema competent to respond to induction by the ureteric bud. PAX2 promotes condensation of the mesenchyme preparatory to tubule formation, while WNT4 causes the condensed mesenchyme to epithelialize and form tubules
Fetal urine is excreted into amniotic sac, where it is swallowed and recycled – Contributes to amniotic fluid volume.
Placenta, not the fetal kidney is responsible for excreting the wastes.
Uretero-pelvic junction is the last to canalize and commonest site of obstruction in congenital hydronephrosis.
Metanephros development
Ureteric bud: Collecting system
- Ureters
- Renal pelvis
- Major calyces
- Minor calyces
- Collecting ducts
Metanephric cap/blastema/mass/mesoderm: Nephron
Metanephric mesoderm → Metanephric vesicles → Primitive S-shaped renal tubules → Nephrons
- Collecting tubules
- Distal convuleted tubule (DCT)
- Loop of Henle
- Bowman’s capsule
Ascent and Rotation of Kidneys
- Fetal metanephros is located at S1-S2
- Definitive adult kidney is located at T12-L3
- The ascent of kidneys is due to disproportionate fetal growth caudal to metanephros.
- During the ascent – kidneys rotate 90°, causing the hilum, which initially faces ventrally, to finally face medially.
- Rather than “drag” their blood supply with them as they ascend, the kidneys send out new and slightly more cranial branches and then induce the regression of the more caudal branches until definitive renal arteries develop at L2.
Ureter and Urinary Bladder
Ureter:
- Originates from ureteric bud
- During 5th week: ureter is patent, probably as a result of the mesonephros producing urine which fills the tube.
- Mid 6th week: ureter loses its lumen.
- Late 6th week: ureter regains a lumen starting at the midpoint and progressing in both directions toward the developing kidney and the urogenital sinus.
The last segments of the ureter to gain a lumen are at either end (kidney or urogenital sinus).
Urinary bladder:
- Cloaca is an endodermal-lined chamber that contacts the surface ectoderm at cloacal membrane. It is connected:
- Anteriorly: To allantois (alongside the vitelline duct)
- Posteriorly: Hindgut
- Cloaca divides by urorectal septum (mesoderm) during 4th-7th weeks.
- Anteriorly connected to allantois: Urogenital sinus
- Posteriorly connected to hindgut: Anal canal
- Upper part of urogenital sinus forms urinary bladder.
- Allantois becomes fibrous cord called urachus or median umbilcal ligament (not medial umbilical ligament; medial umbilical ligament are the remnants of umbilical arteries).
- Trigone is formed by incorporation of lower ends of mesonephric duct (mesoderm) into the posterior wall of bladder as the bladder expands.
- With time, the mesodermal lining of the trigone is replaced by endodermal epithelium, so that finally, the inside of the bladder is completely lined with endodermal epithelium.
Clinical Correlate
Renal agenesis
- Ureteric bud fails to develp → No collecting system and No nephrons (no ureteric bud to induce differentiation of metanephric mesoderm to vesicles and nephrons)
- Unilateral renal agenesis is relatively common (more in male)
- Bilateral renal agenesis: relatively uncommon
- Causes Potter sequence: Decreased amniotic fluid volume (Oligohydramnios) → Fetal compression → facial deformities, limb deformities (club foot) and pulmonary hypoplasia (due to decreased nutrients and alveolar hydrostatic pressure).
- Explains role of amniotic fluid as a cushion to fetus.
Duplication of the urinary tract
- Occurs when the ureteric bud prematurely divides before penetrating the metanephric blastema
- Results in either a double kidney and/or a duplicated ureter and renal pelvis.
- The term duplex kidney refers to a configuration where two ureters drain one kidney.
- The ureter from the lower pole opens normally at the urinary bladder trigone. However, the ureter from the upper pole usually has an ectopic opening.
Renal-Coloboma syndrome
- The Pax2 gene mutations causing:
- Renal hypoplasia
- Vesicouretral Reflux – most likely due to improper connection of the ureter to the bladder or possibly due to inherent defects in epithelial cells of the mature ureter.
- Colobomas – due to failure of the optic fissure to fuse (role of PAX2)
Nephroblastoma (Wilms Tumor)
- Most common renal malignancy of childhood
- Due to deletion of tumor supressor gene WT1 on chromosome 11
- Found in infants from 0-24 months of age
- Consists of blastemal, epithelial, and stromal cell types
- Essentially due to incomplete mesenchymal-to-epithelial transformation (i.e. the cells fail to fully differentiate and transform into cancerous cells)
- Associations: WAGR complex (Wilms tumor, aniridia [absence of the iris], genitourinary malformations, and mental retardation).
Congenital Polycystic Kidney Diseases
They can arise due to a variety of factors:
- loss of polarity: aberrant differentiation of tubule cells results in inappropriate location of Na/K channels to the apical (rather than basal) domain of the cells. Na+ is pumped apically, water follows resulting in dilation of tubule lumens.
- Overproliferation: excessive growth of tubule epithelium can occlude the lumen causing blockage.
Autosomal Recessive Polycystic Kidney Disease (ARPKD):
- Cysts form from Collecting ducts.
- Mapped to the short arm of chromosome 6 (p6).
- Renal failure occurs in childhood.
Autosomal Dominant Polycystic Kidney Disease (ADPKD):
- Cysts form from all the segements.
- Mutation of PKD 1 gene on chromosome 16 or PKD 2 gene on chromosome 4 that encodes Polycystin-1 and 2 respectively.
- Renal failure doesn’t occur until late adulthood.
The interaction of polycystin-1 and polycystin-2 in renal tubules promotes the normal development and function of the kidneys.
Pelvic Kidney
- One or both of the kidneys fail to ascend from pelvic to lumbar region.
- Remains close to common iliac artery.
Horse-shoe Kidney
- Inferior poles of the kidneys fuse across the midline.
- Normal ascent of the kidneys is arrested because the fused portion gets trapped below the origin of the inferior mesenteric artery from the abdominal aorta.
- Kidney rotation is also arrested so that the hilum faces ventrally.
- IVU shows flower-vase appearance.
Supernumerary or Accessory Arteries
- Persistence of embryonic vessels formed during the ascent of the kidneys.
- Usually derived from aorta and enter superior or inferior pole of kidney.
- Often asymptomatic but can sometimes compress the ureter.
Bladder defects
1. Urachal Cyst, Sinus and Fistula:
Due to peristence lumen of allantois, they are found along the midline on a path from the umbilicus to the apex of the urinary bladder (i.e. along median umbilical ligament) –
- Completely: urachal fistula (urine drains from umbilicus)
- Only in the upper part: urachal sinus
- Only local area: urachal cyst
2. Trigonitis: As a mesonephric duct derivative, the trigone is sensitive to sex hormones and can undergo hormone-induced epithelial metaplasia (usually transformation from a transitional type to squamous type epithelium which can overproliferate and lead to urinary blockages).