It is not necessary to memorize each and every step of the process. We will only look into the major events.
A. Meaning: Glyco (Sugar) + Lysis (Breaking or splitting)
B. Synonyms: Embden-Meyerhof Pathway (EM Pathway)
C. Site: Cytoplasm
D. Enzyme basics:
- Kinase: Adds or removes phosphate from substrate (uses ADP or ATP)
- When phosphorylation (ATP used): Enzyme = “Substrate” + Kinase (e.g. Hexokinase)
- When dephosphorylation (ATP produced): Enzyme = “Product” + Kinase (e.g. Pyruvate kinase)
- Dehydrogenase: Oxidizes substrate, reducing electron acceptor (NAD+/NADP+ or FAD or FMN)
- Converts aldehyde to alcohol
E. Key concepts:
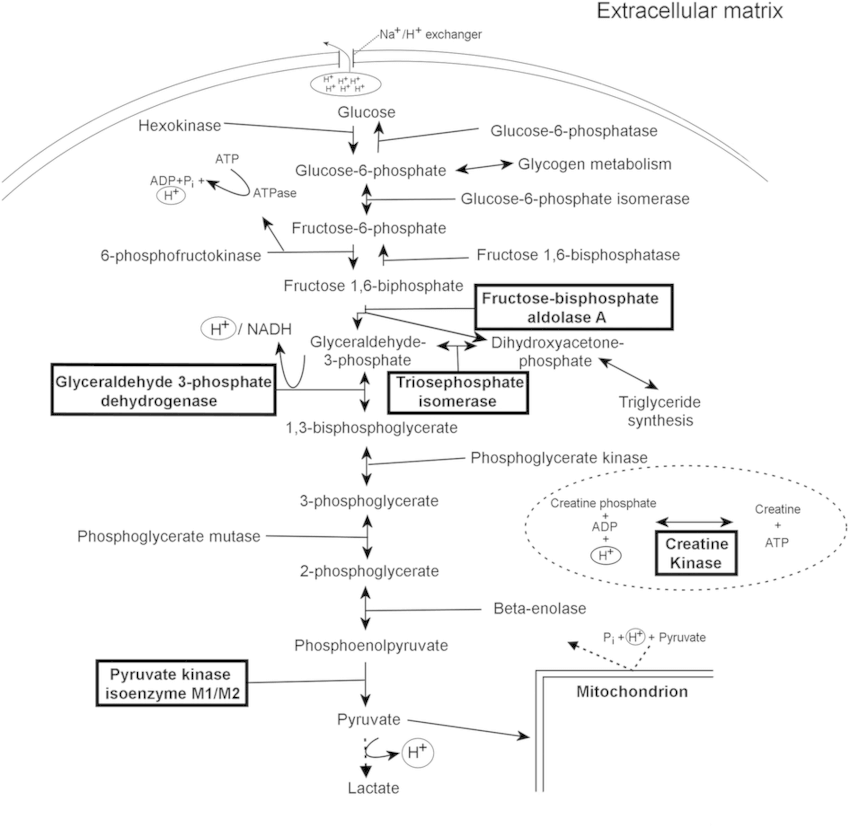
- 1 molecule of glucose (6 C) is converted to 2 molecules of pyruvate (2 X 3C; aerobic) or lactate (2 X 3C; anaerobic).
- During the initial splitting phase (from 6 C to 3 C) during addition of Phosphate to substrate: Energy is used
- After the split of one 6C compound to two 3C compound: 2 molecules are generated (Energy is generated)
- Phosphorylation of substrate consumes ATP: 1 in each step (splitting hasn’t occured)
- Glucose to Glucose-6-Phosphate (Glucokinase/Hexokinase) – 1 ATP
- Fructose-6-Phosphate to Fructose-1,6-Biphosphate (Phosphofructokinase) – 1 ATP
- Dehydrogenation (Oxidative phosphorylation) reaction: uses NAD to generate NADH (2 in each step – splitting has already occured)
- Glyceraldehyde-3-Phsophate (G3P) to 1,3 Biphosphoglycerate (G3P dehydrogenase) + 2 NADH
- Dephosphorylation of substrate yields ATP: 2 in each step (splitting has already occured)
- 1,3 Biphosphoglycerate (1,3 BPG) to 3-Phosphogylcerate (Phosphoglycerate kinase) + 2 ATP
- Phosphoenolpyruvate (PEP) to Pyruvate (Pyruvate kinase) + 2 ATP
- Net ATP gain:
- Aerobic: -1 ATP – 1 ATP + 2 ATP + 2 ATP + 2 NADH = 2 ATP + 2 NADH (1 NADH yields 2.5 ATP in new concept depending upon shuttle or 3 ATP as per old concept) = 7 to 8 ATP
- Anaerobic: -1 ATP – 1 ATP + 2 ATP + 2 ATP = 2 ATP (NAD+ is regenerated from NADH by reduction of pyruvate to lactate by lactate dehydrogenase)
- ATP directly generated from phosphorylation = Substrate level phosphorylation
- Irreversible reactions:
- 3 out of 4 “Kinase” reactions discussed above are irreversible.
- Hexokinase/Glucokinase, Phosphofructokinase and Pyruvate kinase (irreversible due to very large negative ΔG)
- These are potential sites for regulatory control (usually this is because blocks elsewhere in a pathway may lead
to the accumulation of intermediates) and availability of substrate (in this case, glucose), is another general point for regulation. - At each 3 of these points, glycolytic reactions are bypassed by alternate gluconeogenic reactions:
- Hexokinase/Glucokinase by Glucose-6-Phosphatase
- Phosphofructokinase by Fructose 1,6 Biphosphatase
- Pyruvate kinase by Pyruvate carboxylase and PEP carboxykinase
How NADH yields ATP?
- Glycolysis generates NADH in cytosol.
- To make ATP, it must be transferred to electron transport chain inside the mitochondria.
- NADH itself cannot cross inner mitochondrial membrane.
- This is done by malate-aspartate shuttle: Malate IN – Asparate OUT (Malate goes in and is oxidized to Oxaloacetate generating NADH but it also cannot cross the mitochondrial membrane, hence must generate Aspartate by transamination reaction – so that the aspartate goes out to cytosol and regenerates oxaloacetate by transamination reaction)
Glycolysis is aerobic except in following conditions (in which anaerobic glycolysis occurs):
- Limited supply of oxygen: Renal medull
- Few or no mitochondria: RBCs
- Greatly increased demands for ATP: Skeletal muscle during exercise
E. Glucokinase (Hexokinase IV) vs Hexokinase (Hexokinase I-III):
The difference between glucokinase and hexokinase I-III is that glucokinase only works at high glucose concentrations. Liver, the site of glycogen synthesis, has a homologous enzyme called glucokinase. This has a high Km for glucose. This allows brain and muscle to utilize glucose prior to its storage as glycogen
F. Entry of other sugars into glycolysis:
- Fructose:
- Fructose is phosphorylated by fructokinase (liver) or hexokinase (adipose) on the 1 or 6 positions respectively.
- Fructose-6-phosphate is an intermediate of glycolysis.
- Fructose-1-phosphate is acted upon by an aldolase-like enzyme that gives DHAP and glyceraldehyde.
- DHAP is a glycolysis intermediate and glyceraldehyde can be phosphorylated to glyceraldehyde-3-P.
- Glycerol: It is phosphorylated to G-3-P which is then converted to glyceraldehyde 3 phosphate.
- Galactose:
- It has a slightly complicated multi-step pathway for conversion to glucose-1-phosphate.
- gal → gal-1-P → UDP-gal → UDP-glc → glc-1-P.
- If this pathway is disrupted because of defect in one or more enzymes involved in the conversion of gal to glc-1-P, then galactose accumulates in the blood and the subject suffers from galactosemia.
F. Regulation of Glycolysis:
1. Hexokinase:
- Inhibited by accumulation of Glucose-6-Phosphate (feedback inhibition).
- Glucose-6-phosphate is required for other pathways including the pentose phosphate shunt and glycogen synthesis. So hexokinase step is not inhibited unless G-6-P accumulates (no regulation by downstream intermediates/products of metabolism).
2. Glucokinase:
- No direct feedback inhibition as in hexokinase.
- Induced by insulin and blood glucose and inactivated by glycogen.
- Indirectly inactivated by Fructose-6-Phosphate (by inducing protein that binds glucokinase and repress it).
- Consumption of excess fructose can cause an imbalance in liver metabolism by indirectly depleting liver cells of ATP.
3. Phosphofructokinase:
- Rate limiting step
- PFK-1 controls entry of G6P into glycolysis – when the rate of PFK-1 is slowed, G6P accumulates and is routed towards glycogen synthesis or the pentose phosphate pathway.
- PFK-1 is allosterically regulated by:
- Fructose 2,6 Biphosphate: “well-fed” signal that stimulates PFK-1 in liver (synthesized from F6P by PFK-2 when insulin levels are high; glucagon inhibits the same and stimulates fructose biphosphatase-2)
- AMP: stimulates PFK-1 in muscle (inhibits fructose biphosphatase and gluconeogenesis)
- ATP and citrate: slow glycolysis when energy is abundant (activates fructose biphosphatase to form glucose)
- It’s counterpart enzyme (Fructose biphophatase-1) is the rate limiting enzyme in gluconeogenesis.
4. Pyruvate kinase:
- Feed forward stimulation: Fructose 1,6 Biphosphate (prevents metabolic roadblock when PFK is active)
- Allosteric inhibition: ATP and Alanine (biosynthetic product of pyruvate; mobilized from muscle in fasting state) – allows PEP to accumulate and undergo gluconeogenesis
Hormonal regulation:
1. Insulin (well-fed state): Decrease blood glucose (stimulate glycolysis and inhibit gluconeogensis)
- Stimulate glucokinase, PFK and Pyruvate kinase (not hexokinase)
- Inhibit counterpart enzymes of gluconeogenesis, i.e. glucose 6 phosphatase, fructose biphosphatase, Pyruvate carboxylase and PEP carboxykinase
2. Glucagon (starvation): Increase blood glucose (inhibit glycolysis and stimulate gluconeogenesis)
- Inhibit glucokinase, PFK and Pyruvate kinase (not hexokinase)
Fasting state: ↑ glucagon → ↑ cAMP → ↑ protein kinase A → ↑ FBPase-2, ↓ PFK-2
Fed state: ↑ insulin → ↓ cAMP → ↓ protein kinase A → ↓ FBPase-2, ↑ PFK-2
- DAP forms Glycerol-3-Phosphate (G3P) by G3P Dehydrogenase; G3P goes into Triglyceride synthesis and Electron shuttle
- In RBCs: 2-3 BPG mutase form 2,3 DPG from 1,3 BPG which binds to β-chains of HbA and decreases affinity for oxygen – shifts oxygen dissociation curve to right
- Enolase is inhibited by fluoride.
- PK deficiency: Hemolytic anemia, Increased 2,3-BPG, No Heinz bodies (RBCs are without mitochondria and are dependent upon anaerobic glycolysis – PK deficiency leads to decreased ATP leading to:
- loss of biconvex shape which gets destroyed in spleen
- decreased activity of Na+K+ATPase pump resulting in loss of ion balance and causes osmotic fragility
Gluconeogenesis begins 4-6 hours after the last meal and becomes fully active as the stores of liver glycogen are depleted and peaks at 5-7 days of fasting (it is not a source of energy for liver – hepatocytes use beta-oxidation to supply energy needed for gluconeogenesis).
In gluconeogenesis, glucose is synthesized from non-carbohydrate precursors like:
- Glucogenic amino acids (all except leucine and lysine)
- Lactate (produced by muscles during anaerobic glycolysis)
- Glycerol (produced by adipose tissue)
- Propionate (from beta-oxidation of odd chain fatty acid, oxidation of isoleucine and cholesterol side chain)
Liver (major) and kidneys are major gluconeogenic tissues; the kidney contributing upto 40% of total glucose synthesis in fasting state and more in starvation. It doesn’t occur in muscles because muscles lack glucose-6-phosphatase, hence, free glucose cannot be released.
Acetyl CoA (produced by beta-oxidation of fatty acids in liver after lipolysis during fasting state) cannot be used as a substrate for gluconeogenesis but it is an allosteric activator of pyruvate carboxylase (converts pyruvate to oxaloacetate to go into gluconeogenesis) and inhibitor of pyruvate dehydrogenase (converts pyruvate to acetyl CoA to go into TCA cycle), pushing pyruvate towards gluconeogenesis.
Cahill cycle (Alanine-glucose cycle): Glutamate (containing toxic amino group due to muscle protein break down) can be transported to liver by converting it to glutamine or it can transfer it’s amino group to pyruvate (product of glycolysis) by action of ALT (SGPT) to form Alanine which is then transported to liver. In the liver, alanine is deaminated back to pyruvate. Amino group goes into urea cycle and pyruvate goes into gluconeogenesis. Glucose thus formed is transported back to muscle for use.
Cori cycle (Lactic acid cycle):
First, in the direction of lactate formation the LDH reaction requires NADH and yields NAD+ which is then available for use by the glyceraldehyde-3-phosphate dehydrogenase reaction of glycolysis. These two reaction are, therefore, intimately coupled during anaerobic glycolysis. Secondly, the lactate produced by the LDH reaction is released to the blood stream and transported to the liver where it is converted to glucose. The glucose is then returned to the blood for use by muscle as an energy source and to replenish glycogen stores. This cycle is termed the Cori cycle.
In the muscles, glycolysis results in the production of two units of ATP. However, the liver uses up six units of ATP to carry out the process of gluconeogenesis. The Cori cycle also requires the initial introduction of oxygen, without which it cannot begin. As such, eventually, the muscles are bound to require a new supply of glucose as well as oxygen.
If a physical activity is too strenuous, the energy requirements of the muscles will exceed the capacity of the Cori cycle to regenerate glucose from lactate. This will result in a condition known as lactic acidosis.
Enzymes:
Involves both cytosolic and mitochondrial enzymes.
The non-reversible steps in glycolysis must be bypassed with special gluconeogenic enzymes.
1. Pyruvate carobxylase (mitochondrial):
In mitochondria, pyruvate is converted to oxaloacetate via pyruvate carboxylase. But as discussed earlier, inner mitochondrial membrane is not permeable to oxaloacetate, hence, oxaloacetate must be converted to malate (by malate-aspartate shuttle) to exit from mitochondria to cytosol.
Activated by acetyl coA.
2. PEP carboxykinase (mitochondrial and cytosolic):
Activated by Glucagon and cortisol.
3. Fructose 1,6 biphosphatase (cytosolic)
4. Glucose 6 phosphatase (ER of hepatocytes)

He is the section editor of Orthopedics in Epomedicine. He searches for and share simpler ways to make complicated medical topics simple. He also loves writing poetry, listening and playing music. He is currently pursuing Fellowship in Hip, Pelvi-acetabulum and Arthroplasty at B&B Hospital.



Loved it. Just went through it to tevise the concepts. Covered almost everything. Thanx !!
Speech less
Wow whatta content, provided in a nice and structured manner
Thank you sirr