A. Anatomy and Physiology of Body Fluid Compartments:
Remember the “60-40-20” rule of body water.
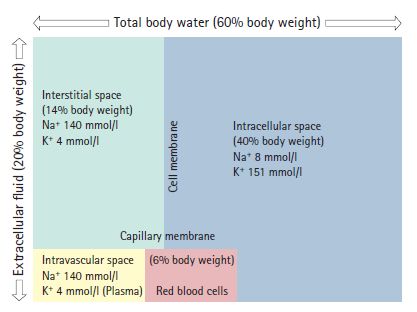
1. Total body water:
- 60% (50-70%) of Total Body Weight
- Greater in lean individuals because fat contains little water
- Greatest percentage in newborns, then decreases with age
2. Intracellular fluid (ICF):
- 40% of Total Body Weight
- Mostly in skeletal muscle mass (thus slightly lower in females than in males)
- Contains large anions like protein and glycogen, which cannot escape and, therefore draw in K+ ions to maintain electroneutrality (Gibbs-Donnan equilibrium). The primary cation of ICF is K+.
3. Extracellular fluid (ECF):
- 20% of Total Body Weight
- Intravascular fluid/plasma (6% of TBW) + Extravascular/interstitial fluid (14% of TBW)
- Primary cation: Na+
- Albumin is the most important oncotic molecule in the ECF. Its concentration in blood (intravascular space) is ~40 g/litre. Transcapillary escape rate of albumin in a healthy individual is 5%/hr, which then returned to the circulation via the lymphatics at the same rate.
- Starling effect: While the hydrostatic pressure within the circulation tends to drive fluid out, the oncotic pressure of the plasma proteins (eg. albumin), draws fluid in and maintains the relative constancy of the plasma volume as a proportion of the ECF.
- In a healthy adult, the total blood volume is ~5L of which 2L are occupied by RBCs and the rest is plasma. In adults the total blood volume can be estimated as 75 ml/kg of body weight.
- 3rd space: It is the space in the body where fluid does not normally collect in larger amounts. Examples include peritoneal and pleural cavity.
Water Movement between ICF and ECF:
Osmolality: It refers to the solute concentration in the body fluid by weight. In humans, normal osmolality in plasma is about 275-295 mOsm/kg. It can be calculated as: Plasma osmolality (Posm) = 2 [Na+] + Glucose/18 + BUN/2.8
Nonisotonic fluid shifts: When the osmolality of either ECF or ICF changes, water moves along the osmotic gradient from the hypotonic compartment to hypertonic compartment until a new osmotic equlibrium is reached.
Isotonic fluid shift: Iso-osmotic fluid gains and losses are distributed only within ECF because without a change in osmolality, water will not shift between compartments.
Fluid and electrolyte balance in 70 kg man:
a. Water balance:
- Intake (2500 ml) = Oral (1500 ml) + Food (750 ml) + Metabolism (250 ml)
- Output (2500 ml) = Urine (1500 ml) + Feces (100 ml) + Insensible loss from skin and lungs (900 ml)
b. Normal plasma concentration of electrolytes:
- Na+: 135-145 mmol/l
- K+: 3.5-5 mmol/l
c. Normal maintenance requirements:
- Water: 25-35 ml/kg/day
- Na+: 1-1.5 mmol/kg/day
- K+: 1-1.5 mmol/kg/day
d. Approximate electrolyte concentration of gastrointestinal and skin secretions: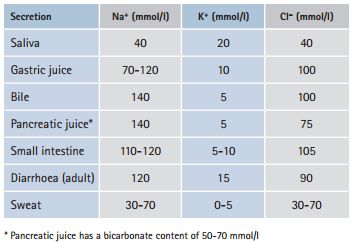
Water requirements increase with: fever, burns, surgical drains, gastrointestinal losses through vomiting or diarrhea, sweating, tachypnea, polyuria. Water requirements increase by 100-150 ml/day for each degree celsius of body temperature elevation.
Physiological changes during stress:
- ↑ADH: Thirst, water retention, potassium loss
- ↑Cortisol: Sodium/water retention, potassium excretion
- ↑Renin-Angiotensin-Aldosterone axis: Sodium reabsorption
- ↑Organ osmo/chemoreceptor activity: Endocrine/sympathetic catabolic state
- ↑Systemic inflammatory response: Cytokine induced capillary leak (Transcapillary albumin excape rate increased to 13-15%/hr which draws water and Na+ into interstitial space resulting in intravascular hypovolemia with edema)
B. Assessment and monitoring of fluid balance:
1. History:
- Fluid deficit: poorly controlled diabetes, vomiting, diarrhea, diuretics, blood loss, burn injury
- Fluid excess: intraoperative fluid
2. Examination: ABCDEF
- Autonomic responses: Pallor, sweating, tachycardia, hypotension
- Blood pressure: falls
- Capillary refill time: slow
- CVP: decreases
- Dry mouth
- Elasticity of skin: decreased skin turgor
- Edema
- Facies: sunken
Notes:
- Decreased CVP/JVP is the 1st indicator of a falling intravascular volume.
- Systolic pressure doesn’t usually fall until 30% of blood has been lost.
- Pedal &/or sacral edema occurs in both volume overload and intravascular volume depletion associate with hypoalbuminemia.
3. Measurements and Investigations:
a. Urine output:
- Fluid infusion is indicated if urine output is <30 ml/hr (<0.5 ml/kg/hr) in the presence of other signs of intravascular hypovolemia. Other signs of dehydration should be looked for because, the oliguria may be a normal physiologic response to the surgery. In such cases, fluid infusion may lead to volume overload.
b. Fluid balance charts:
- Relatively inaccurate and doesn’t take account of insensible losses
- Good measure of changes in urine output, fistula loss, gastric aspirate, etc.
c. Weighing:
- 24 hr recording of weight is pretty accurate
- Takes account of insensible losses
- But weight remains constant in 3rd space losses
d. Invasive monitoring:
- Central venous catheters or Arterial lines can be used in indicated patients
e. Laboratory tests:
- Elevated plasma creatinine/urea/hematocrit/albumin, and low urinary acidity/sodium concentration indicates significant dehydration.
- Plasma lactate/acid base status may highlight metabolic derangement associated with hypovolemia and hypoperfusion.
- Creatinine clearance is a robust measure of renal function and can be estimated at the bedside using Cockcroft and Gault equation:
- Creatinine clearance = 1.23 X (140-age) X weight (kg)/serum creatinine (micromol/l)
- Replace 1.23 with 1.04 for female caluclation
C. Intravenous fluids:
1. Crystalloids:
a. Balanced salt solution (BSS) eg. Hartmann’s (Ringer’s lactate) solution:
- First line replacement therapy in perioperative period.
- Composition: Na+ (131 mEq/l), Cl- (111 mEq/l), K+ (5 mEq/l), Ca2+ (2 mEq/l), HCO3- (29 mEq/l as lactate)
- pH: 6.5
- Osmolarity: 278 mOsm/l (slightly hypotonic)
- Blood should not be given through the same drip set because RL contains calcium.
b. Normal saline 0.9%:
- Commonly used for electrolyte replacement.
- Composition: Na+ (154 mEq/l), Cl- (154 mEq/l)
- May be responsible for hyperchloremic acidosis
- It is preferred over RL for treating:
- Hypochloremic metabolic acidosis
- Brain injury (Ca2+ in RL can increase neuronal injury)
- Hyponatremia
c. Glucose solutions (5% dextrose):
- It contains no electrolytes.
- 5% glucose contains 5 g glucose/100ml (provides energy 4 kcal/g)
- These are isotonic but once glucose is rapidly metabolized, these solutions act as if they are hypotonic.
- Should only be used when there is a risk of overloading the body with sodium.
- Blood cannot be given through the same drip otherwise rouleaux formation will cause clumping of RBCs.
d. Dextrose Normal Saline (DNS):
- Composition: 4% glucose and 1/5 NS (i.e in 1l it contains 40 g glucose and 31 mmol each Na+ and Cl-)
Note:
If crystalloids are used to replace blood, it is necessary to infuse 2-3 times the volume lost as the majority of the fluid given is rapidly transferred to the extravascular compartment as the endothelial barrier is fully permeable to ions in these solutions.
The disadvantages of crystalloid infusions are:
- After blood loss, the hemodynamic inprovement is short-lived
- Peripheral edema
2. Colloids:
Colloids are solutions of large molecules which have an oncotic pressure – the molecules do not normally cross the endothelial barrier and enter the tissues or intracellular spaces.
Types:
- Natural: Human albumin solution, Plasma protein fraction, Fresh frozen plasma, Immunoglobulin solutions
- Semi-synthetic: Gelatins (Gelofusine, Haemaccel), Hydroxyethyl starch, Dextrans
Advantages:
- Reduced tissue edema
- Reduced cerebral edema
- Plasma expander
- Disadvantages:
- Allergic reactions
- Pulmonary edema in cases of capillary leak
- Coagulopathy if more than 1-2 L is infused
- Renal toxicity
- Albumin is expensive
Notes: Although, in theory, colloids that are isooncotic with plasma should expand the blood volume by the volume infused, in practice, the volume expanding capacity of these colloids is only 60-80%.