Master the knowledge of clinically relevant cell cycle to understand the principles of chemotherapy. Tumors that are highly responsive to chemotherapeutic agents (e.g., testicular cancer, lymphomas) tend to have a very rapid doubling time compared to tumors that are less responsive to chemotherapy (e.g., pancreatic and prostate cancers). The tumor doubling time of metastases from any primary tumor tend to be more rapid than the cells found in the primary site.
General principles of Chemotherapy
Can be delivered in a primary, adjuvant, and neoadjuvant fashion.
1. Primary chemotherapy: delivery of chemotherapy alone without any additional therapies such as surgery or radiation therapy (hematologic malignancies such as leukemia and lymphoma).
2. Adjuvant chemotherapy: to a patient who has undergone surgery with curative intent but who is at high risk for relapse (i.e., node-positive breast cancer).
3. Neoadjuvant chemotherapy: given to patients before surgery to “downsize” the tumor to permit a less radical
operation (large breast cancers to allow breast-conserving surgery).
Cells of G0 phase are resistant to chemotherapy.
Mechanism of action:
- Toxic to rapidly growing cells – even normal (GI mucosa, bone marrow)
- Large tumors are relatively unresponsive
- Combination is likely to be effective, if they act on different phases of cell cycle
Non-phase dependent: Alkylating agents, 5-fluorouracil, anthracyclines
- Kills cells exponentially with increasing dose
- Equally toxic for cells within cell cycle
Phase dependent: Methotrexate, Vinca alkaloids
- Kills cells at lower dose
- Act within specific phase of cell cylce
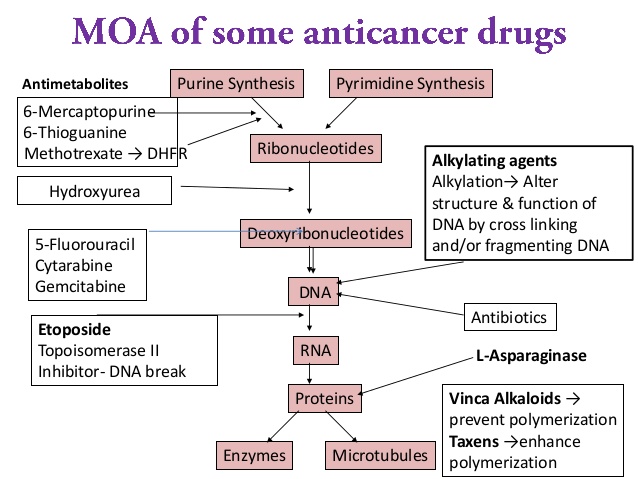
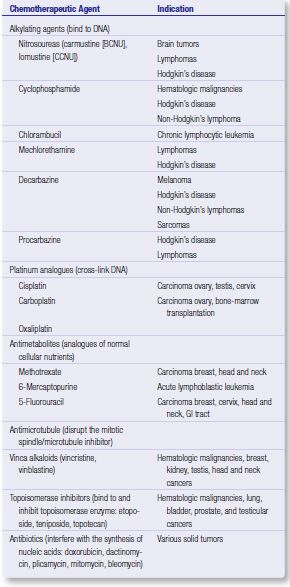
ANTIMETABOLITES
Drugs: Methotrexate, AraC, Gemcitabine, 5-Flourouracil, Capecitabine, Hydroxyurea, Pentostatin, Fludarabine, 2’-Chlorodoxyadenine
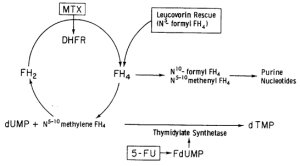
Antifolates (Methotrexate):
- Effects predominant upon actively dividing cells – myelosuppresion and GI mucositis (the 2 organ systems have highest relative cellular turnover in normal tissues)
- Leucovorin rescue: Reduced folate leucovorin administration after 24 hours after chemotherapy allows purine and pyrimidine synthesis to resume in normal tissues, while the more rapidly growing tumor cells die from lack of nucleoside synthesis in the previous 24 hours.
- Other toxicities of methotrexate: Transient hepatoxicity (high doses), Portal cirrhosis (chronic dosing), Methotrexate pneumonitis, Extremity motor paralysis, cranial palsy and seizures (3 or more weeks of intrathecal therapy)
Cytidine analogues (Arabinosylcytosie or AraC, Gemcitabine): Unique class of antimetabolites, that undergoes phosporylation to form nucleoside/nucleotide analogue which will bind to DNA polymerase and be incorporated into growing DNA strand. Besides myelosupression and GI toxicity:
- Gemcitabine can cause prolonged thrombocytopenia
- AraC can cause significant neurologic toxicities like slurred speech, cerebellar ataxia, dementia
Fluorinated pyrimidines (5-Fluorouracil): converted to 5-flourodeoxyuridine (5FDUMP) in the cytoplasm which binds to the enzyme thymidine nucleosides; 5-FU is also incorporated into the synthesis of flourouridine triphosphate (FUTP) which is incorporated into all classes of RNA, which leads to mistakes in translation of proteins and cell death.
- Addition of reduced folates, such as leukovorin, formed more stable tertiary complexes with FDUMP/TS. This combination enhanced cellular toxicity since pyrimidine synthesis could be inhibited.
- Toxicities: Myelosupression, GI toxicity, Hand-foot syndrome (exfoliation of palms and soles), neurotoxicity (UMNL, somnolence, cerebellar ataxia), Cardiotoxicity at high doses
- Disadvantage: short half-life in the plasma secondary to rapid hepatic clearance; can be overcome by 24 hour infusion via central line
- Doxifluidine (Capecitabine) is an oral prodrug that converts into 5-FU in tumor
Hydroxyurea: inhibits the ribonucleotide reductase enzyme system, which is responsible for conversion of ribonucleotides to deoxynucleotides. The net result is depletion of nucleotides which slows DNA synthesis and DNA repair. It is also used as radio-sensitizing agent.
MICROTUBULE INHIBITORS
Microtubules form the mitotic spindles which allow for daughter chromosomal separation in cellular division. Besides, it is also involved in nerve conduction and neurotransmission.
Vinca alkaloids: bind to the heterodimer of tubulin and inhibit microtubule formation. This results in cells arrested in the mitosis phase of cell division.
- Vincristine: Peripheral neuropathy, cranial nerve toxicity and SIADH
- Vinblastine: bone marrow suppression, mucositis, Vinblastine, myalgias (does not cause peripheral neuropathies)
- Navelbine: is a semisynthetic derivative of the vinca alkaloids
Taxanes (Palcitaxel, Docetaxel): prevent the ability to partially or fully depolymerize the mitotic spindle during mitosis
- have limited ability to solubilize in water, they must be mixed in Cremophor and ethanol (risk of anaphylaxis)
- toxicities: neuropathies, neuralgias and bone marrow suppression
- also acts as radio-sensitizing agent
ALKYLATING AGENTS
Interstrand DNA cross-linking, which results in inactivation of the DNA template, inhibition of DNA synthesis and cell death.
Melphalan and chorambucil: used in myeloproliferative disorders; given for four days at intervals of 28 days to allow bone marrow recovery
Cyclophosphamide, Ifosfamide: prodrugs; used in breast cancer and high grade lymphomas
Nitrosoureas: lipophilic; can cross BBB
Toxicities: Myelosuppression, Leukemia, Hemorrhagic cystitis (cyclophosphamide and ifosfamide; infused with uroprotective drug Mesna), Interstitial pneumonitis (Cyclophosphamide), Cardiotoxicity (high doses of cyclophosphamide)
Other drugs: Procarbazine, Dacarbazine
ANTITUMOR METABOLITES
Derived from yeast species Streptomyces
Drugs: Actinomycin D, Mitomycin C, Bleomycin
DACT:
- interrupts transcription of RNA from DNA templates and disrupts synthesis of essential proteins for cellular division.
- for tumors in pediatric oncology including Wilm’s tumor, rhabdomyosarcoma and Ewing sarcoma
- myelosuppression, nausea, diarrhea and hair loss
Mitomycin C:
- behaves like alkylating agent
- active in tumors from lung, breast and gastrointestinal system
- toxicities: leukopenia, thrombopenia, HUS, interstitial pneumonitis
Bleomycin:
- taken up by tumor cells and will bind with iron molecules to form a bleomycin-Fe (II) complex. This complex binds to DNA and results in the generation of oxygen free radicals which lead to DNA strand cleavage.
- lacks significant toxicity of bone marrow function, which allows it to be combined with a wide variety of classic chemotherapeutic agents
- used in Hodgkin’s lymphoma and testicular cancer
- toxicities: interstitial pneumonitis, skin erythema
TOPOISOMERASE INHIBITORS
Topoisomerase I and II mediate ligation and religation reactions for ‘unknotting’ DNA strands in supercoiled DNA which when inhibited leads to breakage of DNA and cell death.
Drugs: Etoposide, Teniposide, Irinotecan, Topotecan, 9-Aminocaptothecin
ANTHRACYCLINES
Drugs: Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mitoxantrone, Platinum analogues (Cisplatin, Carboplatin, Oxaliplatin)
Platinum analogues act like alkylating agents
Cisplatin:
- given under conditions of prolonged intravenous hydration to prevent nephrotoxicity
- acute myelosuppression and nausea, with cumulative effects on hearing and peripheral neuropathies
- Sulfur containing compound amifostine, has been shown to reduce the degree of neuro- and nephrotoxicity of cisplatin.
Carboplatin: spares the patient adverse effects on the renal and neurologic systems.
SIGNAL TRANSDUCTION INHIBITORS
HER2 membrane receptor is an oncogene that is overexpressed in tumors of the breast, prostate and lung
Trastuzumab: Monoclonal antibody against HER2 receptor
R115777: inhibits farnesyl protein transferase preventing franesylation of RAS protein, thus preventing attachment of RAS to cell membrane
Flavopiridol: inhibits CDKs and induces growth arrest in G1 phase
ANTIANGIOGENESIS DRUGS
Drugs: TNP-470, thalidomide, Endostatin, Angiostatin
Reference: Surgical oncology – David N. Krag, M.D.