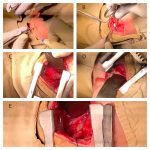
INCIDENCE
- Male > Female
- Age group: 50-75 years
- Second highest cancer in both sexes after prostate (male) and breast (female)
- Smokers > Non-smokers
ETIOLOGY OF LUNG CANCER
A. Cigarette smoking:
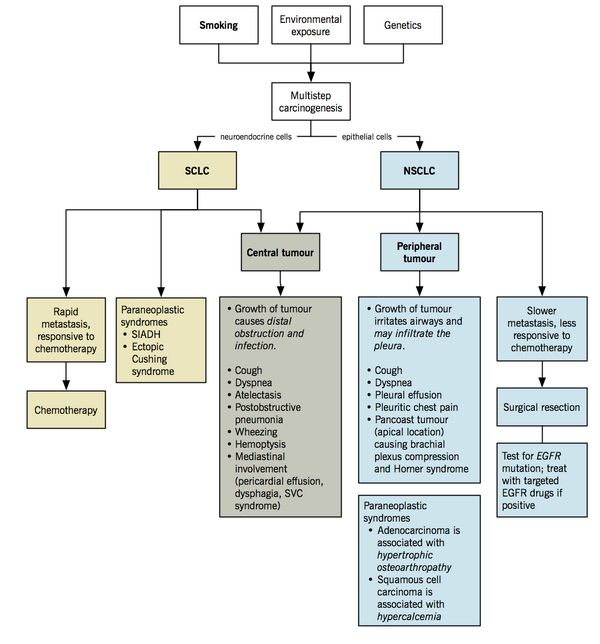
Smoking is the single most important risk factor for lung cancer, which can cause all types of lung cancer but is more strongly linked with Small cell carcinoma and Squamous-cell carcinoma.
Risk of cancer increases with: duration, intensity and depth of smoke inhalation
Type of tobacco smoking: Cigarette smoking (fine particles reaching distal airways) > Pipe and cigar smoking (large particles that only reaches upper airway)
Association: Active smoking > Passive smoking
Important carcinogens:
- Polycyclic Aromatic Hydrocarbons (PAH): e.g. benzopyrene produces p53 mutation (G to T transversion)
- N-nitroso compounds
- Phenol derivatives
Chronic irritation: Leads to squamous metaplasia
Nicotine: Causes addiction and also promotes carcinogenesis
- Sympathetic/Parasympathetic activation: nicotine binds to nicotinic cholinergic receptors, located on both sympathetic and parasympathetic postganglionic neurons – stimulates both sympathetic (increased heart rate and blood pressure) and parasympathetic (intestinal motility, relaxation) systems.
- Addiction: dopamine release from nucleus accumbens, mediating reward and addiction
- Carcinogen: nicotine does not initiate carcinogenesis, but it does promote initiated cells by nicotinic cholinergic receptor signalling in the lungs. Nicotine has been shown to inhibit apoptosis, proliferate cells, and cause angiogenesis in lung tumours.
Smoking cessation: Risk decreases but never returns to non-smoker (never smoker) level
- Smoking initiates carcinogenesis early-on (3-p deletion, p53 mutation)
- 20-25 years required between smoking onset and cancer onset
- Even after smoking cessation, existing initiated cells may progress if another carcinogen carries on process
B. Never-smokers:
Definition: <100 cigarettes in life-time
Accounts for 25% of lung cancers and is distinctly associated with: female cases, east-asian populations, positive family history, adenocarcinoma type, EGFR mutation, and better prognosis
C. Environmental exposures:
a. Occupational exposures: asbestos, tar, soot, metals (arsenic, chromium, and nickel)
b. Atmospheric air pollution
c. Radiation exposure:
- Thoracic radiation therapy
- Indoor radon-222 (radioactive gas that percolates up soil and becomes concentrated inside buildings)
D. Genetics:
Familial predisposition: Increased risk among first degree relatives
Identified gene: CYP1A1 (of Cytochrome P-450 family) – codes for aryl hydrocarbon hydroxylase
- Increased metabolic activation of pro-carcinogens derived from cigarette smoking
E. Precursor lesions:
Squamous dysplasia: Squamous cell carcinoma
Adenomatous hyperplasia: Bronchioalveolar carcinoma (BAC) – a form of adenocarcinoma
Idiopathic pulmonary neuroendocrine cell hyperplasia: Pulmonary carcinoids
COPD, Idiopathic pulmonary fibrosis and tuberculosis are associated with increased risk of lung cancer.
PATHOLOGY OF LUNG CANCER
Major types of lung cancer: Broadly Small cell and non-small carcinoma with different cells of origin, different pathogenic processes and different genetic mutations
- Small cell (oat cell) carcinoma
- mutations in MYC, BCL-2, c-KIT, p53, and RB
- Non small cell carcinoma: mutations in EGFR, KRAS, CD44, and p16
- Squamous cell (epidermoid) carcinoma
- Adenocarcinoma (including Bronchoalveolar carcinoma)
- Large-cell carcinoma
Squamous cell and Small-cell carcinomas are generally centrally (hilar) placed tumors while large cell and adenocarcinoma are peripherally placed.
Neuroendocrine tumors arise from Kulchitsky cells of bronchial mucosa and comprise typical carcinoid, atypical carcinoid and small-cell lung cancer.
CLASSIFICATION OF LUNG CANCER
| Lung cancer type | Location in the lung | Features |
| Adenocarcinoma (40%) | Peripheral | - Commonest cancer in non-smoker
- Female > Male
- Arises from small airway epithelial and type II alveolar cells
- Should test for EGFR mutation for possible targeted therapy
- May appear at site of scarring
- Tend to form glands and secrete mucin
|
| Squamous cell carcinoma (30%) | ⅔ central ⅓ peripheral | - Associated with cigarette smoking
- Arises from large (proximal) airway epithelial cells
- Tend to create obstruction and cause distal atelectasis
- Intrathoracic spread rather than distant metastasis; therefore, best prognosis
|
| Small-cell lung carcinoma (SCLC) (15%) | Central (endobronchial) - Kulchitsky cells are located at bifurcation of small airways
| - Strongest smoking association
- Arises from neuroendocrine (Kulchitsky) cells
- Causes paraneoplastic syndrome: commonly secrete ADH (SIADH) or ACTH (ectopic Cushing syndrome)
- Rapid growth and early distant metastasis (brain, liver, bone), leading to the worst prognosis
|
| Large cell carcinoma(10%) | Peripheral | - Behave similar to adenocarcinomas but the lesions formed tend to be somewhat larger
|
PATHOPHYSIOLOGY AND CLINICAL FEATURES OF LUNG CANCER
| Symptoms | Mechanism and pathophysiology |
| Primary lung lesion symptoms |
| Cough (50-70%) | - Presence of a mass irritates the cough receptors in the airway
- More common in central (hilar) tumors (more receptors)
- Obstruction from central airway could also lead to post-obstructive pneumonia and distal atelectasis
|
| Weight loss (45%) | - Cancer induced lipolysis and proteolysis leads to loss of adipose and skeletal muscle. Protein synthesis is also reduced via a number of mechanisms.
|
| Hemoptysis (25-50%) | - Tumour in the central airway
- Blood vessels resulting from tumour-induced angiogenesis are leaky and tortuous, predisposing them to easy rupture and causing hemoptysis
|
| Dyspnea (25%) | - Extrinsic or intraluminal airway obstruction
- Activation of mechanoreceptors and chemoreceptors in lungs due to cachexia or hypoxemia/acidosis
- Also due to mediastinal involvement
|
| Chest pain (20%) – common in peripheral tumor | - Tumour involving pleural surface causing pleuritic chest pain
- Also due to mediastinal involvement
|
| Mediastinal involvement |
| Superior vena cava syndrome (Obstruction of SVC by tumor; more commin in SCLCl 2-4%) | Impaired venous return (causing edema) from:- Arms: limb edema
- Periorbital area: periorbital edema
- Ocular mucosal membranes: chemosis (visual disturbance)
- Pharynx and larynx: stridor and hoarseness
- Walls of GI tract: dysphagia
- Cerebral veins/Cerebral edema: headache, confusion
- Neck veins: distended, non-pulsatile neck veins
Reduction of cardiac output (inadequate compensation): pre-syncope, syncope Post-azygous obstruction = Features of pre-azygous obstruction + Dilation of veins over abdomen (retrograde flow through azygous via collaterals to IVC) |
| Pericardial effusion and cardiac involvement | - Infiltrate into the pericardium or press on the heart causing pericardial effusion
- Tamponade, arrhythmias or cardiac failure
|
| Pleural effusion (Chest pain and Dyspnea) | - Benign: lymphatic obstruction, post-obstructive pneumonitis, or atelectasis
- Malignant: malignant cells in pleural fluid
|
| Dysphagia | - Middle 1/3rd esophageal compression by enlarged subcarinal lymph nodes
|
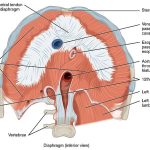
| Pancoast tumour or Pancoast-Tobias tumor (superior sulcus tumour) – superior sulcus or costovertebral gutter extends from 1st rib to diaphragm | - Tumour originates in the apical portion of the lung
- Occurs in 5% of non-small cell lung cancer
- Involves C8 and T1
- Brachial plexus involvement: Motor and sensory complaints in ipsilateral arm
- Sympathetic chain involvement: Horner’s syndrome
- Phrenic nerve involvement: Unilateral diaphragm paralysis
- Recurrent pharyngeal nerve involvement: Hoarseness
|
| Rib pain, pathological fractures and intercostal neuralgia | - Direct extension to chest wall
|
| Paraneoplastic syndromes: symptoms in cancer patients not attributable to tumour compression or invasion (usually result from peptide hormone secretion by tumor) – common with small cell cancer |
| Ectopic Cushing syndrome (most common paraneoplastic syndrome) 1. Rule out pseudo-cushing’s: alcohol excess, major depressive illness, primary obesity 2. Initial tests to diagnose Cushing syndrome: Late-night salivary cortisol level (between 11 PM to midnight) 1 mg overnight 11 PM dexamethasone suppression test (positive if morning 8 AM cortisol >1.8 mcg/dl) 24 hour urinary cortisol (>50 mcg/24 hr) 48 hr 2 mg/low dose dexamethasone suppresion test (0.5 mg 6 hrly started at 9 AM and measured at 9 AM of 3rd day) – 24 hr urine collected from 2nd day and plasma for 3rd day 3. Tests to establish cause: Morning plasma ACTH level(>80 ng/l – ectopic ACTH syndrome or pituitary dependent disease; <10 ng/l – adrenal source) 48 hr High dose 8 mg dexamethasone suppression test (2 mg 6 hrly – positive if cortisol suppression <50% of baseline) – positive in pituitary diseases and negative in ectopic ACTH and adrenal tumors MRI head and Inferior petrosal sinus sampling (Pituitary) CXR, CT chest, Tumor markers (Ectopic ACTH syndrome) | - Ectopic secretion of adrenocorticotrophic hormone (ACTH) → adrenal cortisol secretion → Cushingoid features (Mnemonic: CUSHINGOID)
- Cataracts (posterior subcapsular cataract) – abberant differentiation and migration of epithelial cells; hyperglycemia; G6PD inhibition leading to decreased NADH and glutathione resulting in inhibition of Na/K ATPase pump
- Ulcers (Increased gastric acid secretion and decreased prostaglandin production)
- Skin (plethora, stria, bruising) – thinning of skin due to catabolic effects in epidermis and underlying connective tissue
- Acne: androgen and gluco-corticoid excess
- Hypertension and Hypokalemia – upregulation of RAAS and action on mineralocorticoid receptors resuling in Na+ retention and K+ loss
- Hirsutism – androgen excess by ACTH stimulation
- Hyperglycemia (Diabetic symptoms) – Insulin resistance leading to increased gluconeogenesis, decreased glucose uptake and glycogenesis
- Acanthosis nigricans: Insulin resistance leads to hyperinsulinemia which in turn stimulates proliferation of keratinocytes (containing melanin) and fibroblasts
- Hypercalciuria (Renal stones) – decreased intestinal and tubular reabsorption of calcium
- Hyperlipidemia – Increased lipolysis, VLDL synthesis, fatty accumulation in liver and peripheral insulin resistance
- Hypercoagulability – increased synthesis of fibrinogen and plasminogen activator inhibitor type-1
- Infections of skin (Tinea versicolor)
- Necrosis (avascular) of femoral head – Fat embolization or as a coronary disease of hip (arteriosclerosis)
- Glycosuria – blood glucose exceeds renal threshold (~180 mg/dl)
- Gonadal dysfunction (oligomenorrhea, amenorrhea, impotence) – Inhibition of GnRH, FSH and LH release
- Osteoporosis – Increased RANKL (increases osteoclastogenesis) and decreased osteoprotegrin (decoy receptor for RANKL acting to decrease osteoclast differentiation); Impaired osteoblast differentiation and increased apoptosis; also due to suppresion of gonadotropin and growth hormone, muscle weakness and hypercalciuria
- Proximal myopathy: loss of protein in muscle (negative nitrogen balance) and hypokalemia
- Obesity – Insulin resistance and hyperphagia
- Central obesity (intra-abdominal visceral fat rather than subcutaneous fat): omental fat are able to convert inactive cortisone to cortisol via enzyme (11B-HSD1)
- Moon facies (bitemporal fat deposition)
- Buffalo hump (cervicodorsal fat deposition) – between scapula and neck
- Supraclavicular fat pads
- Immunosuppression (Opportunistic infection) – decreases lymphocyte and monocyte function and production; decreased complement level
- Dernaged mental function (Emotional lability, euphoria, depression, psychosis) – stimulation of mineralocorticoid receptors in limbic system
|
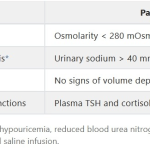
Syndrome of inappropriate antidiuretic hormone production (SIADH)- Hyponatremia (Na <135 mEq/L)
- Hyp0-osmotic plasma (Plasma osmolality <280 mOsm/kg)
- Hyperosmotic urine (Urine osmolality >500 mOsm/kg)
- Hypernatremic urine (Urinary Na >20 mEq/L)
| - Ectopic secretion of ADH → retain free water in collecting ducts
- Euvolemic hyponatremia and concentrated urine
- Mild symptoms (>120 mEq/L): headache, nausea, vomiting
- Moderate symptoms (120-110 mEq/L): restless, irritability, confusion, cramps
- Severe symptoms: include altered mental status, seizures, respiratory depression, and death
- Common in SCLC
|
| Hypercalcemia (>11 mg/dl) and hypophosphatemia | - Increased secretion of PTHrP → acts like parathyroid hormone to increase bone resorption and renal calcium reabsorption → hypercalcemia
- Associated with squamous cell carcinoma
- Clinical features: Stones, Bones, Groans, Moans, Psychic overtones
- Stones – renal stones and nephrocalcinosis; polyuria or polydipsia; impairment of renal function
- Bones – bone pain and fractures; chondrocalcinosis
- Groans – anorexia, constipation, nausea and vomiting, peptic ulcers (calcium increases acidity)
- Moans – fatigue, myalgia, proximal muscle weaness, hypertension (calcified arterial walls), corneal calcification (slit-lamp)
- Psychic overtones – depression, lethargy, memory loss, confusion, coma
- ECG: shortened QT interval, prolonged PR and QRS, AV block
|
| Hypertrophic osteoarthropathy and digital clubbing | - Associated with NSCLC, especially the adenocarcinoma type
- Periosteal proliferation of the tubular bones characterized by (i) painful symmetrical arthritis of the ankles, knees, wrists and elbows, and (ii) digital clubbing.
|
| Dermatomyositis and Polymyositis (Dermatomyositis or polymyositis associated with neoplasia fall under Group III; Others: Group I and II – Idiopathic polymyositis and dermatomyositis respectively; Group IV – Vasculitis associated; Group V – Collagen vascular disease associated) | - Symmetrical proximal muscle weakness
- Muscle biopsy evidence of myositis (muscle necrosis, perifascicular atrophy, lymphocytic infiltration)
- Elevation in serum skeletal muscle enzymes (CK, aldolase, SGOT, SGPT, LDH)
- Characteristic EMG pattern (Myopathic pattern)
- Typical rash of dermatomyositis (Liliac colored Heliotrope rash over upper eyelids)
Definite polymyositis: All of 1-4; Definite Dermatomyositis: 5 + any 3 of 1-4 Other pathognomic cutaneous manifestations: Gottron’s papules (violaceous papules over DIP or MCP areas, elbow or knee), Periungual telangiectasis; Shawl or V sign (erythematous macules distributed in a “shawl” pattern over shoulders, upper chest and neck); Mechanic’s hand (fissured, scaly and hyperkeratotic) |
| Other | - Neuro-myopathic: Lamber-eaton syndrome (pre-synaptic voltage gated calcium channel antibodies; SOX protein antibody; SCLC associated; treated with 3,4-diaminopyridine), Peripheral neuropathy, Subacute cerebellar degeneration, Cortical degeneration
- Hematological: Trosseau’s syndrome (Migratory thrombophlebitis), Marantic endocarditis, DIC, Anemia, Granulocytosis, Leukoerythroblastosis
- Cutaneous: Acanthosis nigricans
- Renal: Nephrotic syndrome (Minimal change disease – benefitted by early use of steroid), Glomerulonephritis
|
| Distant metastasis |
| Metastatic sites include brain, bone, liver and adrenal glands | - Brain: headache, vomiting, neurologic deficits, seizures and confusion
- Bone: pain, pathologic fractures, raised ALP
- Bone marrow: cytopenias or leukoerythroblastosis
- Liver: biochemical liver dysfunction, anorexia, biliary obstruction, pain
- Lymph node: supraclavicular (scalene node)
- Epidural and bone: spinal cord compression syndromes
|