Table of Contents
Definition
Failure of bone marrow to produce peripheral blood cells and its progenitors
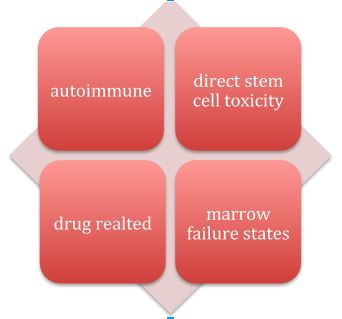
Etiology
The following illustration gives a brief idea about the etiological factors of aplastic anemia
Now going into each etiological factor: –
Autoimmune diseases: – Either they affect all the lineages (autoimmune aplastic anemia) or a single cell lineage (agranulocytosis and pure red cell aplasia).
Causes of autoimmune aplastic anemia: –
- Acquired: – All autoimmune diseases have a chance of affecting the blood cells. It is by the T- lymphocytes suppressing the replicatory activity of the progenitors
- Drugs (discussed below)
- Infections: –
- Hepatitis B: -the evolvement of the anemia is very fast as it affects both the adult cells and the dividing cells.
- EBV: – this viral infection does not directly affect the cells but induces an autoimmune response (molecular mimicry).
Causes of autoimmune single lineage failures: –
- In agranulocytosis: -related to drug usage and autoimmune diseases.
- In pure red cell aplasia: -generally seen in already established cases of AIHA and immunodeficiency diseases when they are infected with Parvo B19 infection.
Direct stem cell toxicity: – whatever the cause is, the cell’s DNA is damaged. The causes are chemicals (benzene) and radiation (depend on dose, time, and part of the body exposed)
Drugs: – theoretically any drug can cause aplastic anemia, as they are foreign to our body. But only some drugs are associated with this. They are classified into classes. They are: –
- Dose dependent: – fluorouracil, chloramphenicol and colchicine are some of the examples
- Dose independent: -hydantoin, quinidine, ranitidine, antithyroid drugs and captopril.
Marrow failure states
- Pregnancy: – generally the outcome is fatal if associated with hemorrhage in pregnancy.
- Paroxysmal nocturnal hemoglobinuria
- Inherited bone marrow failure syndromes: – Fanconi’s anemia, Dyskeratosis congenital and Diamond Blackfan anemia.
Clinical manifestations
Symptoms
Features of anemia: – weakness, easy fatigability and dizziness.
Features of thrombocytopenia: – bleeding and epistaxis.
Features of leucopenia: – recurrent infections.
In females, menorrhagia is seen.
Family history: – information on inherited bone marrow syndromes.
In children parent complains of growth abnormalities
Signs
Pallor
Tachycardia
Petechia and ecchymosis
In children: – growth retardation
Associated renal, cardiac, skin problems and any abnormal fingers may be indicating inherited bone marrow failures.
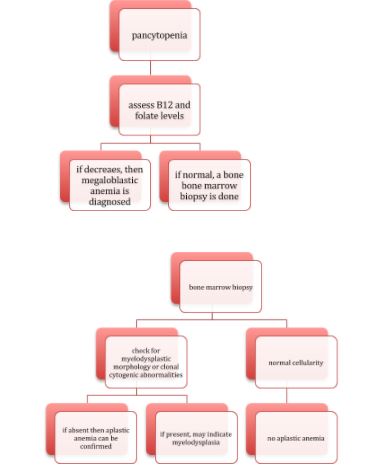
Diagnosis
Depending on the etiological causes discussed above either all the cell counts or only a particular cells counts are decreased.
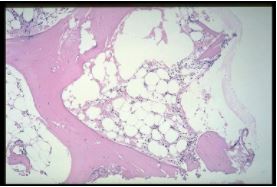
Bone marrow biopsy shows decreased marrow cellularity
But here comes the problem, all the above features may be present in megaloblastic anemia and myelodysplasia.
- Rule out drugs, chemicals and radiation.
- Rule out PNH: – screen for CD55 and CD59.
- Rule out Fanconi’s anemia: – history and chromosome breakage test.
- Rule out Dyskeratosis congenital: – history and flow FISH, telomere length in lymphocyte.
- Diamond Blackfan anemia: – rule out triphalyngeal thumb and gene sequencing are done.
Treatment
Following three modalities are generally followed
- Immunosuppressive therapy
- Stem cell transplant
- Cord blood transplant (not used in congenital marrow failure)
If the patient age is less than 40 years:
| HLA identical sibling | Yes | No |
| Primary treatment | MSBMT | IST |
| Secondary treatment | IST/MSBMT-2 | IST/CBT |
If the patient age is more than 40 years, IST is the only treatment.
MSBMT: -matched sibling bone marrow transplant
IST: – immunosuppressive therapy.
Anti thymocyte globulin: – 12-40mg/Kg/Day for 5 days
Cyclosporine: – 5-10mg/Kg/Day for 6 months
Blood transfusions are done to treat acute attacks or till the treatment formulated starts acting.
Platelet transfusion is given to control life-threatening bleedings.
Treat the infections.
If etiology suggests any drugs, they have to be stopped.
If anemia is due to systemic autoimmune disease, then treating the disease can usually resolve the anemia.
Prognosis: –
Untreated cases generally don’t live a long life.
Younger age patients have a good survival rate, when compared to older population.
Survival rate depends on various factors like the success of the stem cell transplant, age, and general health of the individual and is generally ranging from 75%-85%.
 Author
Author
Sai Venkat
I am a final MBBS student from Telangana, INDIA. I have completed my previous year of MBBS with good percentages with distinctions in microbiology, forensic medicine and ophthalmology.